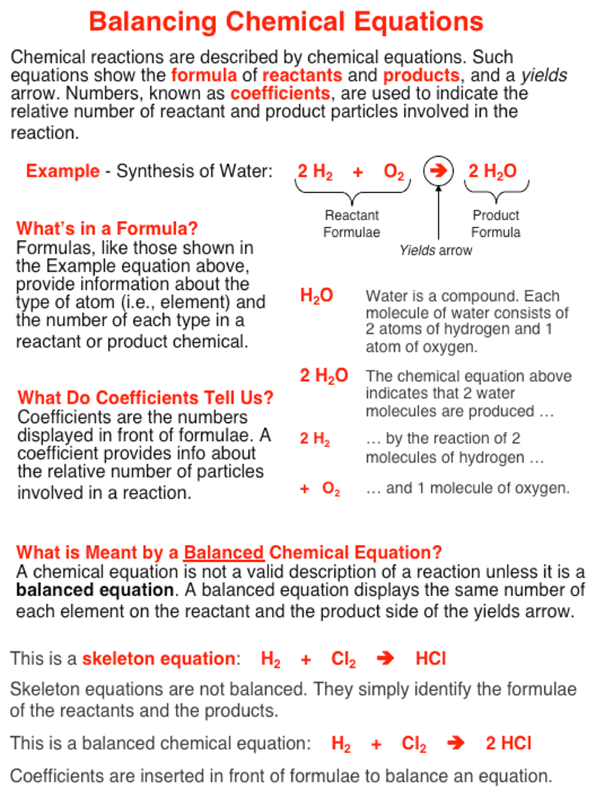
A balanced chemical equation shows the number of particles of each substance involved in a chemical reaction. Such an equation includes formulas and coefficients. The formulas include symbols for each type of atom in the substance. The coefficients are the numbers present in front of the formulas. The act of balancing a chemical equation involves selecting the lowest possible set of coefficients that would provide the same number of atoms of each element on the reactant and the product side of the equation.
Balancing Chemical Equations - Questions 5 Help
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
There are two questions in this Question Group. The two questions are very similar or are of similar difficulty level. The question below is one of the questions.
Version 1:
Identify the lowest possible whole number coefficients that balance the chemical equation.
MnI3 → Mn + I2