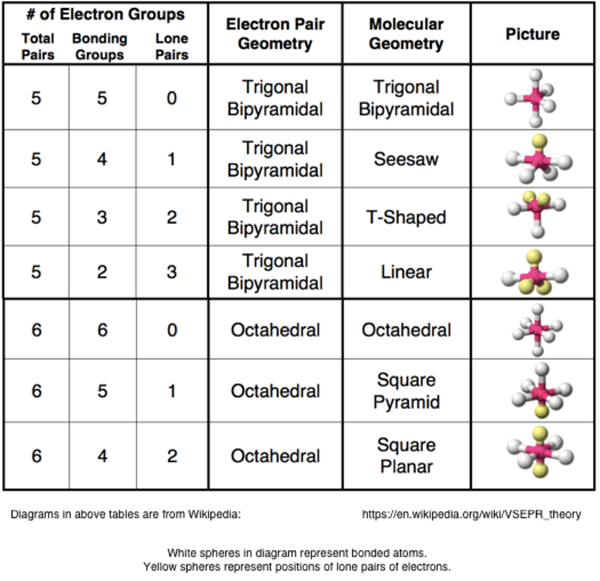
The shape of a molecule can be predicted from the Lewis electron dot diagram. By inspecting the number of groups of electrons AND the number of atoms that surround the central atom of the molecule, the shape of the molecule can be predicted.
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Valence Shell Electron Pair Repulsion Theory - help5
There are three versions of questions in this Question Group. Each version is very similar the others. They only differ in terms of the order in which the molecular formulas are given. Here is one of the versions:
Version 1:
One of these molecules has a shape that is not like the others. Which one doesn't belong? Begin by identifying the correct Lewis electron dot structure. Then identify the one structure that leads to a molecular shape that is different than the other two.
BCl3
NCl3
PF3