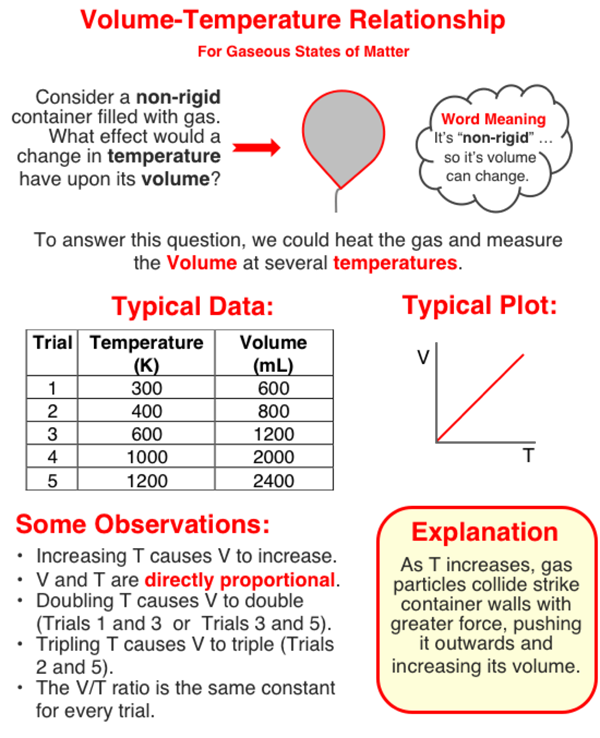
The pressure of a sample of gas is dependent upon the Kelvin temperature of the gas. Increasing the Kelvin temperature increases the pressure. The two quantities are directly proportional to one another. A tripling of the Kelvin temperature will triple the pressure of the gas.
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Pressure and Temperature - help5
There are two questions in this Question Group. Each question is very similar to one another. The question below is one of the questions.
Version 1:
Observe the data table shown at the right for a sample of gas that has a constant volume and number of particles. Use the data table to answer the next two questions.
When the Kelvin temperature of the gas is tripled (increased by a factor of three), the pressure of the gas becomes _____.
a. three times larger
b. six times larger
c. nine times larger
d. one-third the size
e. one-sixth the size
f. one-ninth the size
g. Not possible to tell
Which pairs of trials demonstrate this relationship? Select all that apply.
a. 1 and 2
b. 1 and 3
c. 1 and 4
d. 1 and 5
e. 2 and 4
f. 2 and 5
g. 3 and 4
h. 3 and 5