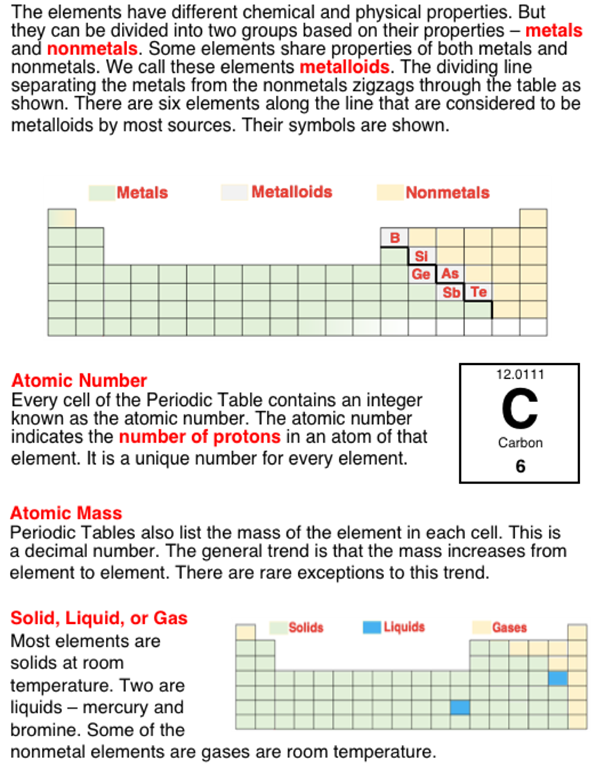
The 100+ existing elements are organized into groups (columns) and periods (rows) of a table known as the Periodic Table. The familiar looking shape of the table is based upon a number of observations about the elements. One of those properties is the property of mass. There is a general trend (that is broken on only a couple occasions) that the atomic mass of an element is seen to increase as one progresses across a row and down a column. If you can find a periodic table that has mass information printed on it, you can inspect the values to see the general patter and you can also find the couple of occasions in which the pattern is broken.
Another property is the property of acting like a metal or a nonmetal. The metal elements are grouped together on the table. The same is true of the nonmetal elements. One grouping of metals is known as the Transition Metals. The transition metal block includes the 10 columns of three rows of elements stretching from Group 3 to Group 12.
Learn more about these ideas in the How to Think About This Situation section below.
Getting your Trinity Audio player ready...
Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Name That Element - help10
There are two similar questions in this Question Group. Each time one of the versions appears, the answer options are presented in a scrambled order. One of the two questions is shown below.
Version 1:
I am a fourth period transition metal. And when you look at my mass (instead of my atomic number), you would expect me to be located one element to the left. Who am I?
Chromium (Cr)
Cobalt (Co)
Copper (Cu)
Iron (Fe)
Manganese (Mn)
Nickel (Ni)
Vanadium (V)
Zinc (Zn)