Help for Wizard Difficulty Level
Water has a weak tendency to dissociate into ions - the hydronium ion (H
3O
+) and the hydroxide ion (OH
-). The tendency of water to form these ions is so weak that the concentrations of ions in solution is very small. A pH scale is used as an indicator of the hydronium ion concentration. It is a logarithmic scale that is commonly used in science and math to express the magnitude of very large and very small numbers. Because the pH scale is a logarithmic scale, a low pH value is an indicator of a higher hydronium ion concentration. The equation that expresses the relationship between pH and hydronium ion (H
3O
+) is ...
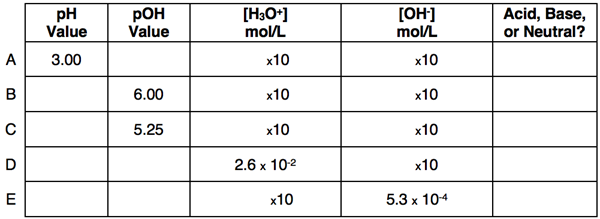
pH = - log ([H3O+])
To determine the hydronium ion concentration ([H
3O
+]) from a pH value requires some algebra performed on the above equation. The result of the algebra manipulation is the following equation.
[H3O+] = 10-pH
A pOH scale is also used to indicate the concentration of the other ion - the hydroxide ion (OH
-). The equations relating the pOH and the [OH
-] are similar to those for the pH scale. They are ....
pOH = - log ([OH-])
[OH-]= 10-pOH
At a temperature of 25°C, the product of these two ion concentrations is approximately 1.0 x 10
-14. This fact is the basis of the pH-pOH relationship, at least for temperatures of 25°C. The pH value and the pOH value add up to 14.
pH + pOH = 14 (at 25°C)
A neutral solution has the same pH value as its pOH value. So a pH of 7 corresponds to a neutral solution. Any pH value less than 7 corresponds to an acidic solution and any pH value greater than 7 corresponds to a basic solution.