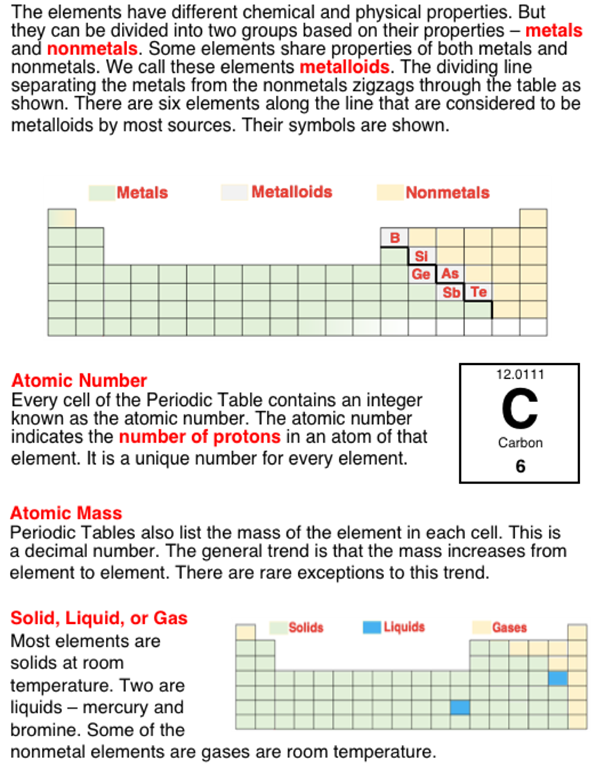
The 100+ existing elements are organized into groups (columns) and periods (rows) of a table known as the Periodic Table. The familiar looking shape of the table is based upon a number of observations about the patterns in the properties and behavior of elements. One of the patterns is the pattern of atomic number. Atomic number refers to the number of protons in an atom of a given element. The elements are organized by atomic number. This is the integer that is typically printed in each cell of the table.
Another property is the property of acting like a metal or a nonmetal. The metal elements are grouped together on the table. There are at least six elements that are difficulty to group with metals or with nonmetals because they tend to have properties of each. We refer to these elements as metalloids. Their location on the table reflects this idea, as they are located between metals and nonmetals along a zig-zag line that starts in Group 13 between Period 2 and Period 3 and continues to the bottom of the chart.
A final property of significance to this question is the property of mass. There is a general trend (that is broken on only a couple occasions) that the atomic mass of an element is seen to increase as one progresses across a row and down a column. So elements located further to the right along a period or further down along a group tend to be more massive (with only a couple of exceptions).
Learn more about these ideas in the How to Think About This Situation section below.
Name That Element - Questions 11 Help
There are two similar questions in this Question Group. Each time one of the versions appears, the answer options are presented in a scrambled order. One of the two questions is shown below.
Version 1:
I have five less protons than the least massive metalloid in the fourth period. Who am I?
Chlorine (Cl)
Cobalt (Co)
Copper (Cu)
Gallium (Ga)
Iron (Fe)
Nickel (Ni)
Phosphorus (P)
Sulfur (S)