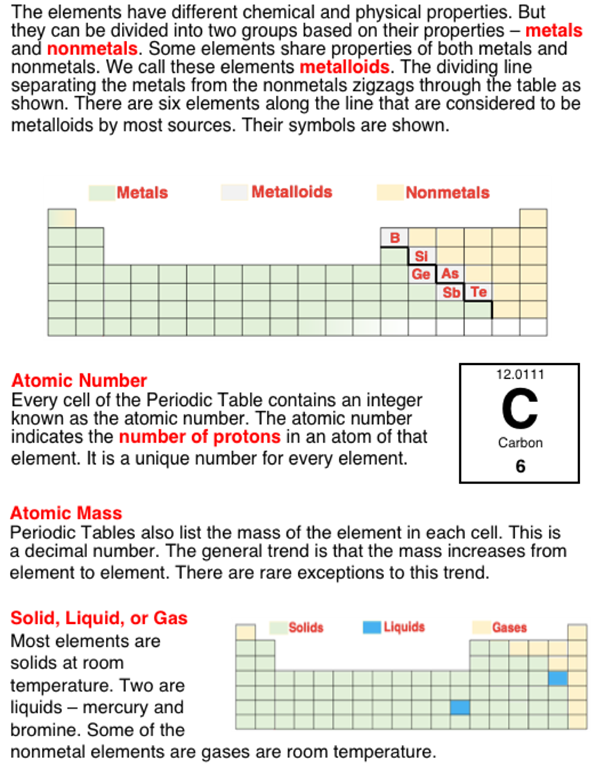
The 100+ existing elements are organized into a table known as the Periodic Table. The familiar looking design of the table conveys information about a number of patterns in the behavior and properties of the elements. One of the patterns is the pattern of atomic number. Atomic number refers to the number of protons in an atom of a given element. The elements are organized by atomic number. This is the integer that is typically printed in each cell of the table. Another property is the property of acting like a metal or a nonmetal. The metal elements are grouped together on the table. The same is true of the nonmetal elements. The two most popular metal families are the Alkalii Metals of Group 1 and the Alkaline Earth Metals of Group 2 (a Group is a column). One grouping of metals is known as the Transition Metals. The transition metal block includes the 10 columns of three rows of elements stretching from Group 3 to Group 12.
Name That Element - Questions 7 Help
There are two similar questions in this Question Group. Each time one of the versions appears, the answer options are presented in a scrambled order. One of the two questions is shown below.
Version 1:
I am the transition metal that has four more protons than the alkali metal found in period 4. Who am I?
Chromium (Cr)
Dubnium (Db)
Manganese (Mn)
Rutherfordium (Rf)
Scandium (Sc)
Thorium (Th)
Titanium (Ti)
Uranium (U)
Vanadium (V)