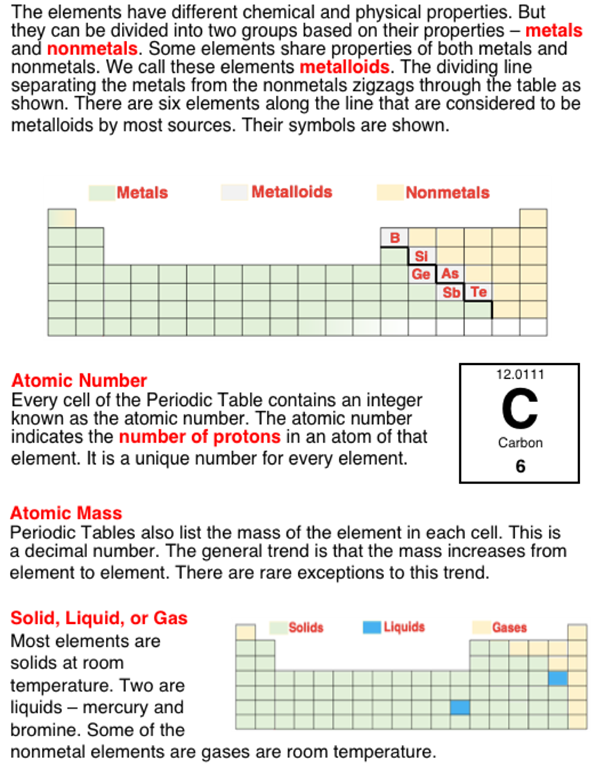
The 100+ existing elements are organized into groups (columns) and periods (rows) of a table known as the Periodic Table. The familiar looking shape of the table is based upon a number of observations about the elements. One of those observations was that there were groupings of elements that had very similar properties. These elements were placed in the same column, referred to as families, and given family names such as alkali metals, alkaline earth metals, halogens, and noble gases. The familiar looking design of the table also conveys information about a number of patterns in the behavior and properties of the elements. One pattern is that melting points and boiling points tend to increase as one proceeds from element to element down a group. In one group - the halogen group - one observes elements that are gases (near top), liquids (more in the middle), and solids (near bottom). Many Periodic Tables will color code the elements based on their solid, liquid, and gas nature. Learn more about these ideas in the How to Think About This Situation section below.
Name That Element - Questions 9 Help
There are two similar questions in this Question Group. Each time one of the versions appears, the answer options are presented in a scrambled order. One of the two questions is shown below.
Version 1:
Compared to the other elements in my family, I am the only halogen that is a liquid at room temperature. Who am I?
Astatine (As)
Bromine (Br)
Chlorine (Cl)
Iodine (I)
Krypton (Kr)
Mercury (Hg)
Xenon (Xe)
To learn more about the law of reflection for plane mirrors, visit the following page at The Physics Classroom Tutorial:
The Law of Reflection