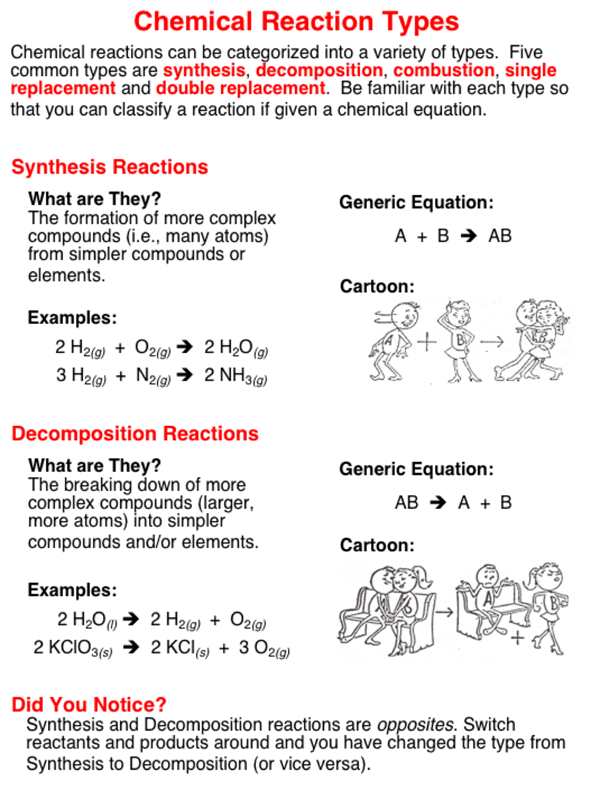
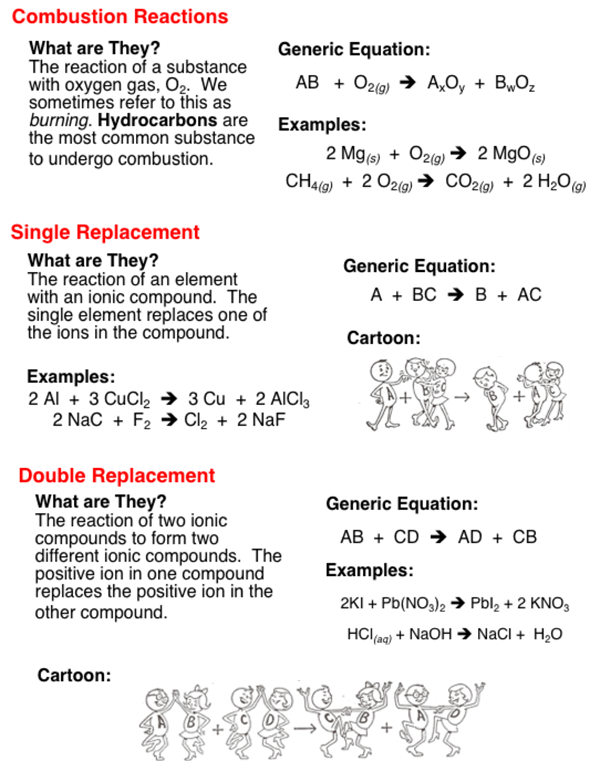
Chemical reactions involve changes - changes of reactants into products. Basic chemical reactions can be grouped into categories based on the types of changes that are occuring during the reaction. There are five basic categories - synthesis, decomposition, combustion, single replacement, and double replacement.
Chemical Reaction Types - Questions 6 Help
There is only one question in this Question Group. But each time the question appears, the ordering of answers is scrambled. Here is the question.
The Question:
Which one these statements describes how to typically tell the difference between a synthesis (S) reaction and a decomposition (D) reaction?
An S reaction has one product and two or more reactants and a D reaction has one reactant and two or more products.
An S reaction has one reactant and two or more products and a D reaction has one product and two or more reactants.
An S reaction has oxygen as a reactant and a D reaction has oxygen as a product.
An S reaction has oxygen as a product and a D reaction has oxygen as a reactant.