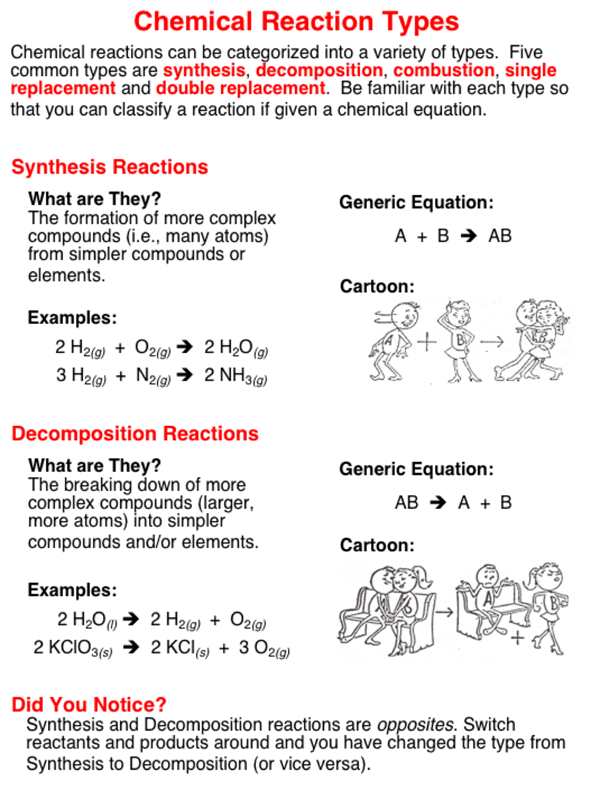
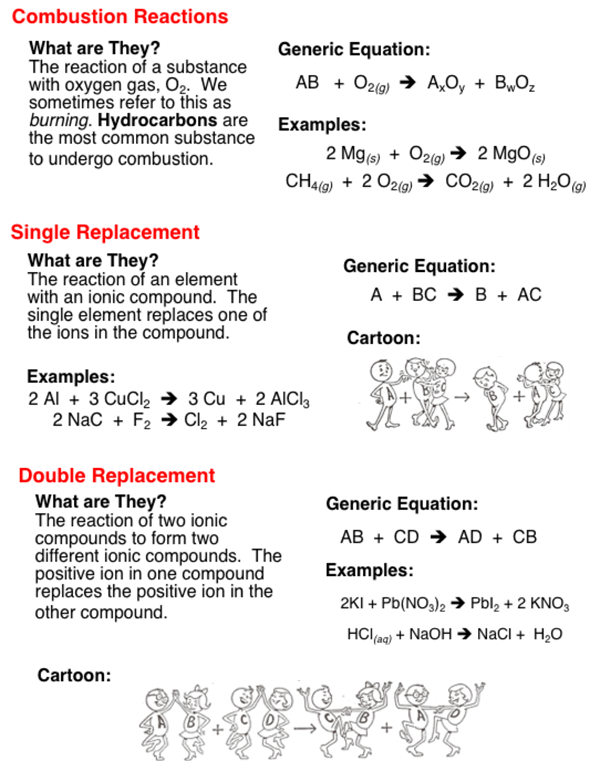
Chemical reactions involve changes - changes of reactants into products. Basic chemical reactions can be grouped into categories based on the types of changes that are occuring during the reaction. There are five basic categories - synthesis, decomposition, combustion, single replacement, and double replacement.
Chemical Reaction Types - Questions 8 Help
There are two questions in this Question Group. The two questions are very similar or are of similar difficulty level. The question below is one of the questions. Each time the questionsi appear, the ordering of multiple choice answer options is scrambled.
Version 1:
Consider the synthesis of magnesium oxide from its elements:
2 Mg + O2 ==> MgO
This reaction could also be categorized as a ______ reaction type.
Decomposition
Combustion
Single Replacement
Double Replacement