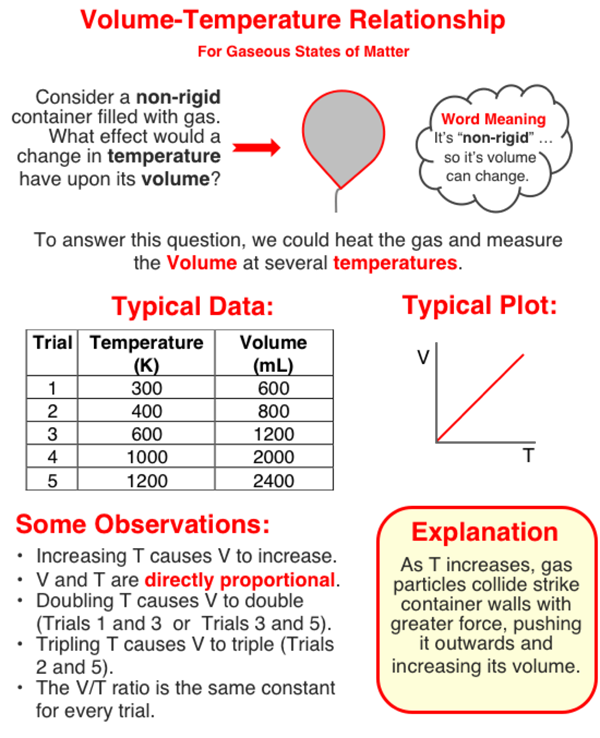
The volume of a sample of gas is dependent upon the Kelvin temperature of the gas. Increasing the Kelvin temperature increases the volume. The two quantities are directly proportional to one another.
Volume and Temperature - Questions 1 Help
There are three questions in this Question Group. Each question is very similar to one another. The question below is one of the questions.
Version 1:
A sample of gas has a constant pressure and number of particles. For such a gas, the relationship between the volume and the Kelvin temperature is best described as a _______ relationship
direct
inverse
quadratic