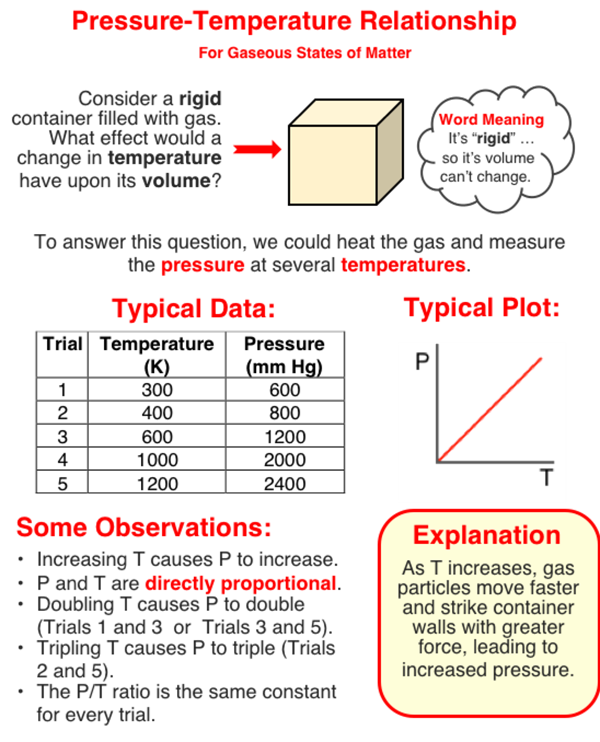
The pressure of a sample of gas is dependent upon the Kelvin temperature of the gas. Increasing the Kelvin temperature increases the pressure. The two quantities are directly proportional to one another.
Pressure and Temperature - Questions 1 Help
There are three questions in this Question Group. Each question is very similar to one another. The question below is one of the questions.
Version 1:
A sample of gas has a constant volume and number of particles. For such a gas, the relationship between the pressure and the Kelvin temperature is best described as a _______ relationship
direct
inverse
quadratic