We have previously learned that visible light waves consist of a continuous range of wavelengths or frequencies. When a light wave with a single frequency strikes an object, a number of things could happen. The light wave could be absorbed by the object, in which case its energy is converted to heat. The light wave could be reflected by the object. And the light wave could be transmitted by the object. Rarely however does just a single frequency of light strike an object. While it does happen, it is more usual that visible light of many frequencies or even all frequencies is incident towards the surface of objects. When this occurs, objects have a tendency to selectively absorb, reflect or transmit light certain frequencies. That is, one object might reflect green light while absorbing all other frequencies of visible light. Another object might selectively transmit blue light while absorbing all other frequencies of visible light. The manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. In this section of Lesson 2 we will discuss how and why light of certain frequencies can be selectively absorbed, reflected or transmitted.
Visible Light Absorption
Atoms and molecules contain electrons. It is often useful to think of these electrons as being attached to the atoms by springs. The electrons and their attached springs have a tendency to vibrate at specific frequencies. Similar to a tuning fork or even a musical instrument, the electrons of atoms have a natural frequency at which they tend to vibrate. When a light wave with that same natural frequency impinges upon an atom, then the electrons of that atom will be set into vibrational motion. (This is merely another example of the resonance principle introduced in Unit 11 of The Physics Classroom Tutorial.) If a light wave of a given frequency strikes a material with electrons having the same vibrational frequencies, then those electrons will absorb the energy of the light wave and transform it into vibrational motion. During its vibration, the electrons interact with neighboring atoms in such a manner as to convert its vibrational energy into thermal energy. Subsequently, the light wave with that given frequency is absorbed by the object, never again to be released in the form of light. So the selective absorption of light by a particular material occurs because the selected frequency of the light wave matches the frequency at which electrons in the atoms of that material vibrate. Since different atoms and molecules have different natural frequencies of vibration, they will selectively absorb different frequencies of visible light.
Visible Light Reflection and Transmission
Reflection and transmission of light waves occur because the frequencies of the light waves do not match the natural frequencies of vibration of the objects. When light waves of these frequencies strike an object, the electrons in the atoms of the object begin vibrating. But instead of vibrating in resonance at a large amplitude, the electrons vibrate for brief periods of time with small amplitudes of vibration; then the energy is reemitted as a light wave. If the object is transparent, then the vibrations of the electrons are passed on to neighboring atoms through the bulk of the material and reemitted on the opposite side of the object. Such frequencies of light waves are said to be transmitted. If the object is opaque, then the vibrations of the electrons are not passed from atom to atom through the bulk of the material. Rather the electrons of atoms on the material's surface vibrate for short periods of time and then reemit the energy as a reflected light wave. Such frequencies of light are said to be reflected.
Where Does Color Come From?
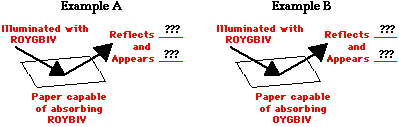
The color of the objects that we see is largely due to the way those objects interact with light and  ultimately reflect or transmit it to our eyes. The color of an object is not actually within the object itself. Rather, the color is in the light that shines upon it and is ultimately reflected or transmitted to our eyes. We know that the visible light spectrum consists of a range of frequencies, each of which corresponds to a specific color. When visible light strikes an object and a specific frequency becomes absorbed, that frequency of light will never make it to our eyes. Any visible light that strikes the object and becomes reflected or transmitted to our eyes will contribute to the color appearance of that object. So the color is not in the object itself, but in the light that strikes the object and ultimately reaches our eye. The only role that the object plays is that it might contain atoms capable of selectively absorbing one or more frequencies of the visible light that shine upon it. So if an object absorbs all of the frequencies of visible light except for the frequency associated with green light, then the object will appear green in the presence of ROYGBIV. And if an object absorbs all of the frequencies of visible light except for the frequency associated with blue light, then the object will appear blue in the presence of ROYGBIV.
ultimately reflect or transmit it to our eyes. The color of an object is not actually within the object itself. Rather, the color is in the light that shines upon it and is ultimately reflected or transmitted to our eyes. We know that the visible light spectrum consists of a range of frequencies, each of which corresponds to a specific color. When visible light strikes an object and a specific frequency becomes absorbed, that frequency of light will never make it to our eyes. Any visible light that strikes the object and becomes reflected or transmitted to our eyes will contribute to the color appearance of that object. So the color is not in the object itself, but in the light that strikes the object and ultimately reaches our eye. The only role that the object plays is that it might contain atoms capable of selectively absorbing one or more frequencies of the visible light that shine upon it. So if an object absorbs all of the frequencies of visible light except for the frequency associated with green light, then the object will appear green in the presence of ROYGBIV. And if an object absorbs all of the frequencies of visible light except for the frequency associated with blue light, then the object will appear blue in the presence of ROYGBIV.
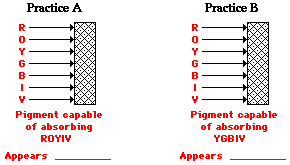
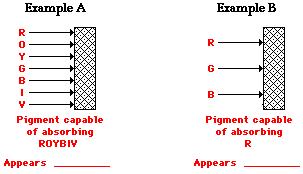
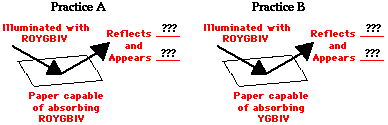
Consider the two diagrams below. The diagrams depict a sheet of paper being illuminated with white light (ROYGBIV). The papers are impregnated with a chemical capable of absorbing one or more of the colors of white light. Such chemicals that are capable of selectively absorbing one or more frequency of white light are known as pigments. In Example A, the pigment in the sheet of paper is capable of absorbing red, orange, yellow, blue, indigo and violet. In Example B, the pigment in the sheet of paper is capable of absorbing orange, yellow, green, blue, indigo and violet. In each case, whatever color is not absorbed is reflected.
 Check your understanding of these principles by determining which color(s) of light are reflected by the paper and what color the paper will appear to an observer.
Check your understanding of these principles by determining which color(s) of light are reflected by the paper and what color the paper will appear to an observer.
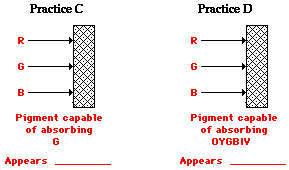
Transparent materials are materials that allow one or more of the frequencies of visible light to be transmitted through them; whatever color(s) is/are not transmitted by such objects, are typically absorbed by them. The appearance of a transparent object is dependent upon what color(s) of light is/are incident upon the object and what color(s) of light is/are transmitted through the object.
 Express your understanding of this principle by filling in the blanks in the following diagrams.
Express your understanding of this principle by filling in the blanks in the following diagrams.
The colors perceived of objects are the results of interactions between the various frequencies of visible light waves and the atoms of the materials that objects are made of. Many objects contain atoms capable of either selectively absorbing, reflecting or transmitting one or more frequencies of light. The frequencies of light that become transmitted or reflected to our eyes will contribute to the color that we perceive.
We Would Like to Suggest ...

Sometimes it isn't enough to just read about it. You have to interact with it! And that's exactly what you do when you use one of The Physics Classroom's Interactives. We would like to suggest that you combine the reading of this page with the use of our
Stage Lighting Interactive. The Interactive is found in the Physics Interactives section of our website and allows a learner to explore the appearance of actors upon the stage when illuminated with varying combinations of red, green, and blue light.
Check Your Understanding
1. Natural philosophers have long pondered the underlying reasons for color in nature. One common historical belief was that colored objects in nature produce small particles (perhaps light particles) that subsequently reach our eyes. Different objects produce different colored particles, thus contributing to their different appearance. Is this belief accurate or not? __________________ Justify your answer.
2. What color does a red shirt appear when the room lights are turned off and the room is entirely dark? ____________ What about a blue shirt? ____________ ... a green shirt? ____________
3. The diagrams depict a sheet of paper being illuminated with white light (ROYGBIV). The papers are impregnated with a chemical capable of absorbing one or more of the colors of white light. In each case, determine which color(s) of light are reflected by the paper and what color the paper will appear to an observer.
4. The appearance of a transparent object is dependent upon which color(s) of light is/are incident upon the object and which color(s) of light is/are transmitted through the object. Express your understanding of this principle by determining which color(s) of light will be transmitted and the color that the paper will appear to an observer.