Interactive - Kinetics and Equilibrium
See how modifying things such as temperature and pressure, and reactants can make an impact.
Activities
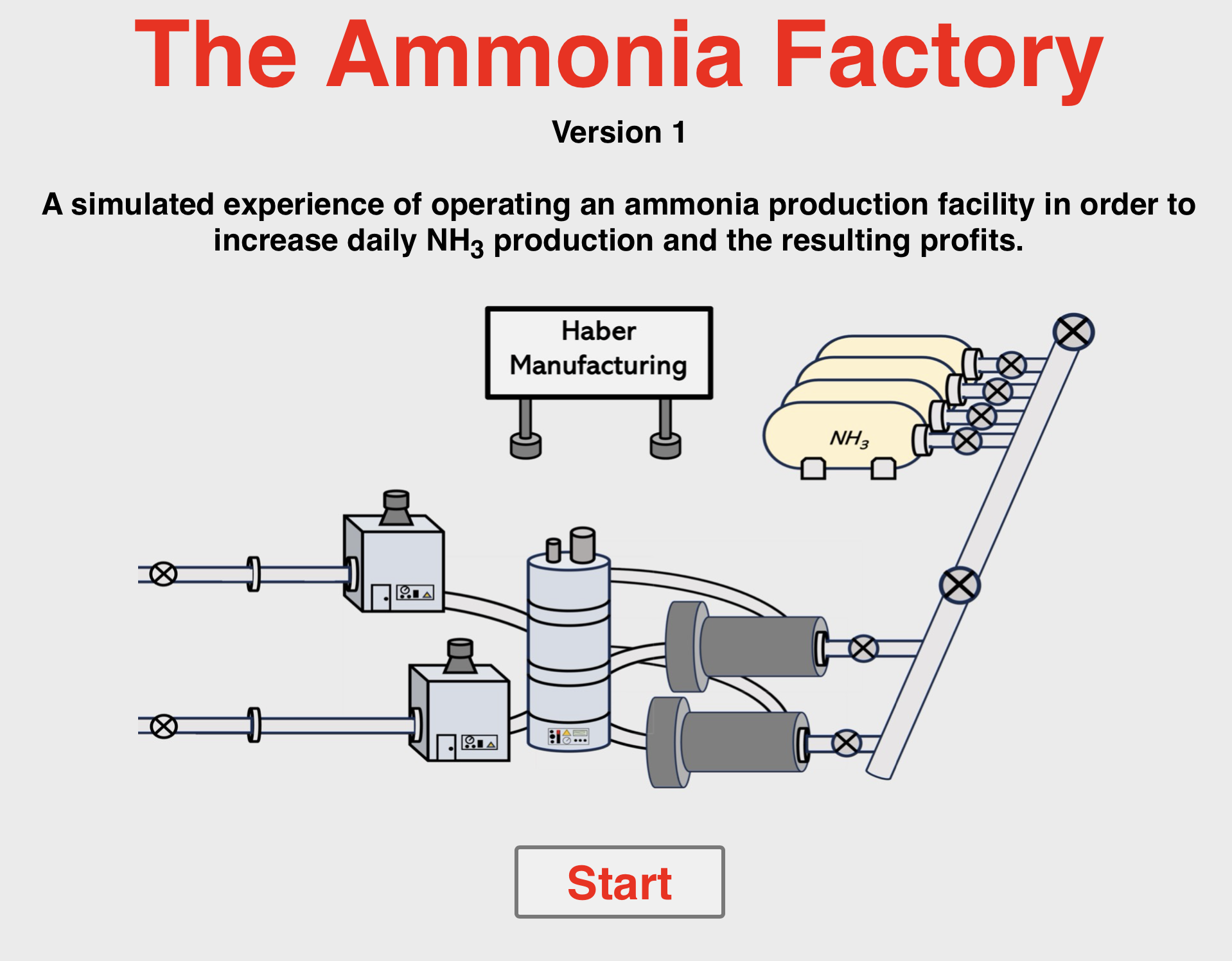
You've been assigned the job of running the production line at the ammonia factory. Your goal is to identify the design parameters that will increase the daily yield of ammonia. You can modify reactor temperatures and pressures and the nitrogen and hydrogen flow rates and observe the effect upon percent yield, daily ammonia production, your carbon footprint, and safety risks. Get started quick because your job ends in 180 days. Good luck!
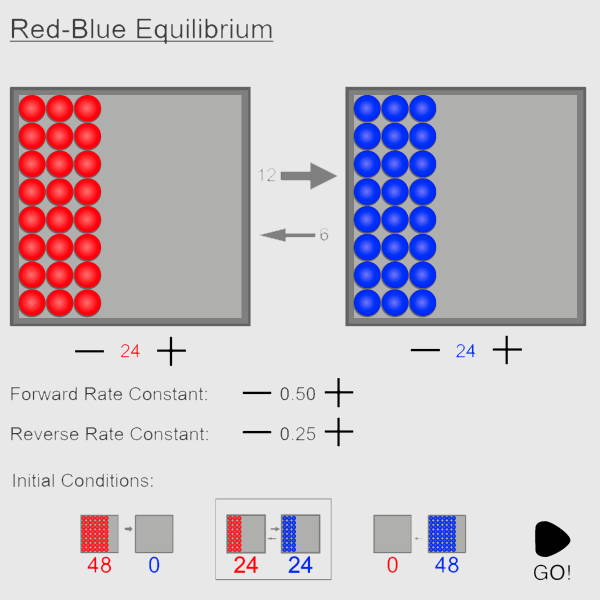
Reversible systems always reach an equilibrium state, regardless of the starting conditions. But what is true of this equilibrium state? How can it be recognized? And how does the system respond if the equilibrium state is disturbed by the addition or removal of reactant or product?
The Physics Classroom would like to thank Nerd Island Studios for contributing this Interactive to our collection.