Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Properties of Liquids
Part c: Surface Tension
Part a:
Boiling and Melting
Part b:
Vapor Pressure
Part c: Surface Tension
Part d:
Capillary Action
Part e:
Viscosity
The Big Idea
This lesson explains how surface tension is a property of liquids that makes their surfaces act like stretched elastic sheets, arising from the unbalanced intermolecular forces at the liquid’s surface. The result is water droplets, beading behavior, and phenomena like paperclips floating or insects walking on water.
What is Surface Tension?
Have you ever noticed drops of rainwater forming beads on top of the hood of a newly waxed car? Or have you noticed water bulging above the rim of an overfilled glass? Or perhaps you have noticed the near spherical drops of water formed from a dripping faucet? Each of these phenomena are an illustration of water exhibiting the property of surface tension.
Molecules on the surface of water (and throughout the bulk of water) experience strong intermolecular forces due to hydrogen bonding. At the surface, a water molecule does not experience these intermolecular forces from all sides since there are no water molecules above them. Surface molecules are pulled sideways and downwards but not upwards. Because of this imbalance of intermolecular forces, surface molecules form a convex shape to reduce the number of molecules on its exterior surface.
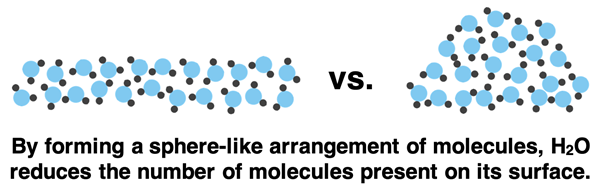
 Surface tension is the property of a liquid that causes its surface to behave as if it were a stretched sheet of elastic material. Surface tension results from the cohesive forces between molecules at the surface of a liquid. Cohesion means to stick together or to be united as a whole. When you see a liquid like water displaying surface tension, you are observing the cohesiveness of its molecules. They’re sticking together! External forces are no match when water molecules are united as a whole by its strong hydrogen bonding forces.
Surface tension is the property of a liquid that causes its surface to behave as if it were a stretched sheet of elastic material. Surface tension results from the cohesive forces between molecules at the surface of a liquid. Cohesion means to stick together or to be united as a whole. When you see a liquid like water displaying surface tension, you are observing the cohesiveness of its molecules. They’re sticking together! External forces are no match when water molecules are united as a whole by its strong hydrogen bonding forces.
The Water Strider
 The photo at the right shows water striders on top of the surface of a pond. (Image Source: Wikimedia Commons) Water striders are known for their unusual ability to walk or even dart across the surface of water. Their long and slender legs allow them to distribute their weight across a large area. As we learned in our Gases Chapter of this Chemistry Tutorial, a force spread over a larger area reduces the pressure on the surface. The legs also have the quality of being hydrophobic. That is, they tend to repel water more than they attract water. Thus, water strider legs won’t absorb water and get weighed down by water absorption. When these features are combined with water’s tendency to act as a stretched elastic sheet, it isn’t difficult to understand why water striders can walk on water. For a water strider, this is Chemistry for Better Living.
The photo at the right shows water striders on top of the surface of a pond. (Image Source: Wikimedia Commons) Water striders are known for their unusual ability to walk or even dart across the surface of water. Their long and slender legs allow them to distribute their weight across a large area. As we learned in our Gases Chapter of this Chemistry Tutorial, a force spread over a larger area reduces the pressure on the surface. The legs also have the quality of being hydrophobic. That is, they tend to repel water more than they attract water. Thus, water strider legs won’t absorb water and get weighed down by water absorption. When these features are combined with water’s tendency to act as a stretched elastic sheet, it isn’t difficult to understand why water striders can walk on water. For a water strider, this is Chemistry for Better Living.
You can do a water strider experiment at home. All you need is a cup of water and some paper clips. A pair of tweezers would also help. Find a location that can get wet; a kitchen or bathroom is a good choice. Fill the cup with water. Using tweezers, carefully place a paper clip flat on the surface of the water. For best results, avoid puncturing water’s stretched elastic sheet with the leading edge of the paper clip or the tweezers. How many paper clips can you balance on the water’s surface? Water is supporting the weight of the paper clip by surface tension. Strong hydrogen bonds hold unite the water molecules. External forces like the weight of the paper clip are no match for this stretched elastic sheet.
Beading
 The photo at the right shows beads or drops of water forming on the surface of a leaf. (Photo by Stockcake.) We refer to the tendency of water to form these sphere-like drops as beading. Beading is another demonstration of water’s high surface tension. Like the water strider, the leaf is hydrophobic or water-repelling. There is very little attraction between the water molecules and the molecules of the leaf surface. The forces between molecules of two different materials is known as adhesion. (We will talk about adhesion in greater detail on the next page of Lesson 2.) In the case of the leaf and water, adhesion forces are very small. Cohesion forces are very strong due to the strong hydrogen bonding forces between water molecules. Cohesion wins. Water molecules stick together and form the bead as opposed to spreading across the surface of the leaf.
The photo at the right shows beads or drops of water forming on the surface of a leaf. (Photo by Stockcake.) We refer to the tendency of water to form these sphere-like drops as beading. Beading is another demonstration of water’s high surface tension. Like the water strider, the leaf is hydrophobic or water-repelling. There is very little attraction between the water molecules and the molecules of the leaf surface. The forces between molecules of two different materials is known as adhesion. (We will talk about adhesion in greater detail on the next page of Lesson 2.) In the case of the leaf and water, adhesion forces are very small. Cohesion forces are very strong due to the strong hydrogen bonding forces between water molecules. Cohesion wins. Water molecules stick together and form the bead as opposed to spreading across the surface of the leaf.
You can do beading experiments at home. All you need is a water dropper, a small amount of wax paper, and a countertop that can get wet. (A fine water mister is a good alternative to a water dropper.) In your kitchen or bathroom, use the water dropper to release one drop of water onto the countertop from about 6 inches above it. Does the water bead up or spread out? Place some wax paper on the countertop and repeat with a drop of water released from 6 inches above it. Does the water behave any differently on the countertop as opposed to the wax paper? Beading is more prevalent on hydrophobic surfaces. The absence of adhesion forces means that cohesion are the main forces influencing the water drop’s behavior.

Drop Formation
The tendency of water to form drops is commonly observed at the faucet. A drop of water forms a spherical shape, seemingly defying gravity as it holds onto the faucet’s surface. Once more, water sticks together. Cohesive forces (hydrogen bonding) pull the water drop into a sphere. The surface of the water drop acts as a stretched sheet of elastic material, gathering more and more molecules of water until finally it weighs too much and the drop surrenders to gravity. Once more, the tendency of water to form these spherical drops is a testimony to its strong surface tension.
 Mercury
Mercury
Mercury is a unique metal. It is the one metallic element whose interparticle forces are not strong enough to result in a solid state metal at room temperature. It is the only metal element that is a liquid. Yet the London dispersion forces between its atoms are strong enough to cause a significant surface tension. Mercury exhibits a high degree of beading due to these strong intermolecular forces.
Mercury also forms a unique meniscus when placed in a glass column like a barometer or a thermometer. While adhesion forces between water and glass cause water to form a concave meniscus, mercury forms a convex meniscus. It has little attraction to the glass and thus very little adhesion to its surface. Cohesion forces dominate and mercury forms a convex meniscus. (We will discuss adhesion, capillary action, and the formation of a meniscus in detail on the next page of Lesson 2.)
Photo Source: Wikimedia Commons
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Surface Tension. Save it to a safe location and use it as a review tool.
Check Your Understanding of Surface Tension
Use the following questions to assess your understanding of surface tension. Tap the Check Answer buttons when ready.
1. Is surface tension a property that is unique to water or do other substances possess this property?
2. Describe the difference between cohesion and adhesion.
3. Hexane, C
6H
14, consists of nonpolar molecules. Predict what would happen if you attempted to balance paper clips on the surface of hexane.