Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Colligative Properties
Part b: Boiling Point Elevation
Part a:
Vapor Pressure Lowering
Part b: Boiling Point Elevation
Part c:
Freezing Point Depression
The Big Idea
When a solute is added to a solvent, the boiling point of the solution rises. This effect, known as boiling point elevation, is a colligative property that depends only on the number of solute particles.
What is Boiling Point Elevation?
 We learned about colligative properties on the previous page of Lesson 3. Colligative properties are properties of solutions that are independent of the identity of the solute and dependent upon the number of particles of solute in the solution. Vapor pressure lowering was the colligative property discussed in Lesson 3a. Adding solute to a solvent causes the vapor pressure of the resulting solution to be less than that of the pure solvent. And the more solute particles that are present in the solution, the more that the vapor pressure will be lowered below that of the pure solvent.
We learned about colligative properties on the previous page of Lesson 3. Colligative properties are properties of solutions that are independent of the identity of the solute and dependent upon the number of particles of solute in the solution. Vapor pressure lowering was the colligative property discussed in Lesson 3a. Adding solute to a solvent causes the vapor pressure of the resulting solution to be less than that of the pure solvent. And the more solute particles that are present in the solution, the more that the vapor pressure will be lowered below that of the pure solvent.
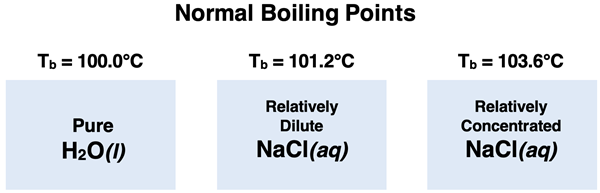
In Lesson 3b, our focus is on a second colligative property known as boiling point elevation. Adding solute to a solvent like water causes the boiling point of the resulting solution to be greater than that of the pure solvent. And the more particles of solute that are in the solution, the more that the boiling point will be increased above that of the pure solvent. While the normal boiling point of pure water is 100°C, adding a solute like NaCl will cause the solution to boil above 100°C. The more solute that is added, the higher the boiling point will be elevated.
The Meaning of a Boiling Point
 We discussed boiling point and its relationship to vapor pressure in Lesson 2b of Chapter 11 of our Chemistry Tutorial. We learned that the vapor pressure of a liquid like water varies with temperature. The data at the right shows water’s vapor pressure for a variety of temperatures.
We discussed boiling point and its relationship to vapor pressure in Lesson 2b of Chapter 11 of our Chemistry Tutorial. We learned that the vapor pressure of a liquid like water varies with temperature. The data at the right shows water’s vapor pressure for a variety of temperatures.
Boiling point is defined as the temperature at which the vapor pressure of water is equal to the atmospheric pressure. The definition implies that the boiling point of a substance is dependent upon the surrounding pressure conditions. While water will boil at 100.00°C at standard pressure conditions (1 atm or 760 mm Hg), its boiling point will be less at lower pressures and greater at higher pressures. This is a consequence of the fact that the vapor pressure depends on temperature as shown in the table of data.
We can reason from this data that water will boil at the following temperatures for the specified atmospheric pressures.
- At Patmosphere = ~55 mm Hg, Tboiling = 40 °C
- At Patmosphere = ~355 mm Hg, Tboiling = 80 °C
- At Patmosphere = ~634 mm Hg, Tboiling = 95 °C
- At Patmosphere = ~760 mm Hg, Tboiling = 100 °C
Explaining Boiling Point Elevation
We learned in
Lesson 3a that the vapor pressure of a solution is less than the vapor pressure of a pure solvent. Because boiling points are associated with vapor pressures, it is natural to expect that dissolved solute particles will also affect the boiling point. Their presence elevates the boiling point. Here is how to unpack the logic behind the principle.
The graph below displays vapor pressure as a function of temperature for a sample of pure water and for an aqueous solution of NaCl (~1.0 M). Observe that for any given temperature, the vapor pressure of water is lowered by the presence of the NaCl. At an atmospheric pressure of 760 mm Hg, pure water will boil at 100.0°C since its vapor pressure is 760 mm Hg at that temperature. But the ~1.0 M aqueous solution will not boil at that temperature since its pressure is less that atmospheric pressure. The solution must be heated to 101.2°C before its vapor pressure matches the atmospheric pressure of 760 mm Hg.
Dependence on the Number of Solute Particles
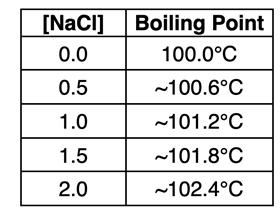
As with any colligative property, boiling point elevation values depend on the number of solute particles present in the solution. The table at the right shows estimates of the boiling point for varying concentrations of NaCl. As the concentration of the solute increases, there are more particles (ions) present in the solution. This in turn has a greater impact on how much the boiling point elevates above the usual 100.0°C.
Sodium chloride (NaCl) is an ionic solute that dissociates into two ions.
NaCl(s) → Na+(aq) + Cl-(aq)
For the same concentration, its effect upon the boiling point would be twice as great as a molecular solid like glucose or sucrose (C
12H
22O
11). A dissociated cation (Na
+) and anion (Cl
-) are counted as two particles. But when considering the number of solute particles present in solution, a dissolved C
12H
22O
11 molecule is counted as a single particle since it does not dissociate.
C12H22O11(s) → C12H22O11(aq)
Finally, an ionic solid like sodium phosphate (Na
3PO
4) dissociates into three cations and one anion.
Na3PO4(s) → 3 Na+(aq) + PO43-(aq)
For the same concentration, sodium phosphate would have four times the boiling point elevation (∆T
b) as sucrose and two times the boiling point elevation as sodium chloride.
Boiling Point Elevation Equation
The boiling point elevation for a solution is given by the equation:
∆Tb = i • Kb • molality
The
∆Tb is the difference between the boiling point of the solution (
Tb-solution) and the boiling point of the pure solvent (
Tb-pure solvent).
∆Tb = Tb-solution - Tb-pure solvent
The
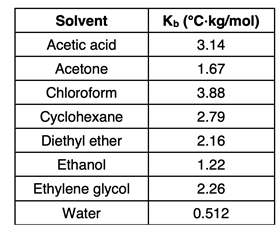
Kb in the equation is referred to as the
boiling point elevation constant. It is a value that depends upon the solvent. A table of values for several common solvents is shown at the right.
The
i in the equation is known as the Van't Hoff factor. For molecular solids that don’t dissociate, the value is 1. For ionic solids like NaCl that dissociate into two ions, the value is 2. For ionic solids like CaCl
2 that dissociate into three ions, the value is 3. For ionic solids like Na
3PO
4 that dissociate into four ions, the value is 4.
The molality is a concentration expression. It is defined as the moles of solute per kilogram of solvent.
Example 1 - Calculation of a Solution's Boiling Point
A student dissolves 5.00 g of calcium chloride in 100.0 mL of water. What would be the expected boiling point of this solution?
Solution
The plan will involve using
∆Tb = i • Kb • molality to determine the increase in boiling temperature. We will then add this ∆T
b value to 100.00°C to determine the boiling point.
As a first step, we will determine the molality of the solution. We will calculate the moles of solute from the mass of solute. We will calculate the mass of solvent from the volume.
Moles solute = 5.00 g CaCl2 • (1 mol CaCl2 / 110.98 g CaCl2)
Moles solute = 0.04505316 … mol CaCl2
Mass of solute = 0.100 L • (1.00 kg/L) = 0.100 kg
Molality = (0.04505316 … mol of ions) / (0.100 kg) = 0.04505316 … mol/kg
As a second step, we will calculate the boiling point elevation using the K
b of water - 0.512°C•kg/mol. The value of i is 3.
∆Tb = i•Kb•molality = 3•(0.512°C•kg/mol) • (0.04505316 … mol/kg)
∆Tb = 0.6920165 … °C
Finally, we can calculate the boiling point of water by adding the ∆T
b to 100.00 °C.
Tb-solution = 100.00°C + 0.6920165 … °C
Tb-solution = 100.69°C
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Download our Study Card on Colligative Properties. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding of Boiling Point Elevation
Use the following questions to assess your understanding of how the addition of a solute increases the boiling point of a solution. Tap the Check Answer buttons when ready.
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Explain why boiling point elevation is considered a colligative property.
2. If (just supposing) the vapor pressure of a solvent increased as more and more solute was added to it, then what affect would this have on the boiling point?
3. Rank the following samples in order of increasing boiling point, from lowest to highest. (Assume an atmospheric pressure of 760 mm Hg.)
- Sample A: Pure Water
- Sample B: 0.25 M C6H12O6(aq)
- Sample C: 0.25 M KI(aq)
4. Rank the following samples in order of increasing boiling point, from lowest to highest. (Assume an atmospheric pressure of 760 mm Hg.)
- Sample A: 1.00 M NaCl(aq)
- Sample B: 0.10 M NaCl(aq)
- Sample C: 0.40 M NaF(aq)
5. Rank the following samples in order of increasing boiling point, from lowest to highest. (Assume an atmospheric pressure of 760 mm Hg.)
- Sample A: 1.00 M KCl(aq)
- Sample B: 1.00 M CuCl2(aq)
- Sample C: 1.00 M AlCl3(aq)