Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 4: Nuclear Radiation - The Good, the Bad, and the Ugly
Part b: Radiation Exposure - The Bad
Part a:
Nuclear Technologies - The Good
Part b: Radiation Exposure - The Bad
Part c:
Nuclear Accidents - The Ugly
The Big Idea
Radiation is invisible, but its effects are measurable. By detecting radiation and quantifying exposure, scientists can assess risk, ensure safety, and apply nuclear technology responsibly.
The Bad Side of Radiation
 In the first three lessons of Chapter 19, we addressed the fundamentals of nuclear stability, nuclear radiation, nuclear decay, and nuclear reactions. In Lesson 4, we are exploring the good, the bad, and the ugly side of radiation. The bad news about radiation is that it is all around us ... at every moment ... of every day ... no matter where you go. In fact, it is even inside you. But that’s not really bad news because it is absolutely normal that radiation is so pervasive and yet our bodies are quite capable of withstanding such exposures. But the bad news can get worse and you will want to learn how and why it gets worse and what you can do about it. In Lesson 4b, we will explore radiation sources - both natural and artificial. And we will learn about how we measure it and about when exposure levels begin to impact health.
In the first three lessons of Chapter 19, we addressed the fundamentals of nuclear stability, nuclear radiation, nuclear decay, and nuclear reactions. In Lesson 4, we are exploring the good, the bad, and the ugly side of radiation. The bad news about radiation is that it is all around us ... at every moment ... of every day ... no matter where you go. In fact, it is even inside you. But that’s not really bad news because it is absolutely normal that radiation is so pervasive and yet our bodies are quite capable of withstanding such exposures. But the bad news can get worse and you will want to learn how and why it gets worse and what you can do about it. In Lesson 4b, we will explore radiation sources - both natural and artificial. And we will learn about how we measure it and about when exposure levels begin to impact health.
Detecting Radiation: Knowing It’s There
 Radiation is invisible and undetectable by human senses. But it’s not a ghost in the closet. It is detectable using specialized instruments such as Geiger counters. When ionizing radiation, the kind that we are concerned about, interacts with matter, it ionizes the matter. Radiation detectors rely upon this fact in order to count the amount of radioactive particles and rays. A Geiger counter is filled with an inert gas like argon (Ar) which is ionized upon contact by an alpha or beta particle. The gas releases an electron, which is registered by the Geiger counter as a “count”. Most Geiger counters display the amount of radiation as the number of counts, as the counts per second, or as the counts per minute.
Radiation is invisible and undetectable by human senses. But it’s not a ghost in the closet. It is detectable using specialized instruments such as Geiger counters. When ionizing radiation, the kind that we are concerned about, interacts with matter, it ionizes the matter. Radiation detectors rely upon this fact in order to count the amount of radioactive particles and rays. A Geiger counter is filled with an inert gas like argon (Ar) which is ionized upon contact by an alpha or beta particle. The gas releases an electron, which is registered by the Geiger counter as a “count”. Most Geiger counters display the amount of radiation as the number of counts, as the counts per second, or as the counts per minute.
Image: Wikimedia Commons
Measurement Units for Radiation Dose
The most standard unit used to express the amount of radiation that a person is exposed to is the sievert (abbreviated Sv). The sievert provides a quantitative measure of the biological risk or damage associated with a particular exposure. Since 1 Sv is a very large dose, millisieverts (mSv) and microsieverts (µSv) are more commonly used. A millisievert is one-thousandth of a sievert. A microsievert is one-millionth of a sievert.
 Another unit that is commonly used, especially in the United States, is the rem. The ill-effects of radiation are due to its ability to ionize body tissue. Different forms of radiation have different ionizing ability. The rem unit (short for Roentgen equivalent man) considers both the dosage amount and the type of radiation. Alpha particles, with their greater mass, have a significantly greater ionizing ability than light-weight beta particles and gamma rays. The rem accounts for this fact. The rem is an older unit that has since been replaced by the sievert. One sievert is equivalent to 100 rem.
Another unit that is commonly used, especially in the United States, is the rem. The ill-effects of radiation are due to its ability to ionize body tissue. Different forms of radiation have different ionizing ability. The rem unit (short for Roentgen equivalent man) considers both the dosage amount and the type of radiation. Alpha particles, with their greater mass, have a significantly greater ionizing ability than light-weight beta particles and gamma rays. The rem accounts for this fact. The rem is an older unit that has since been replaced by the sievert. One sievert is equivalent to 100 rem.
Background Radiation
 As we stated earlier, radiation is all around us at every moment of every day no matter where we are. Radiation is natural and inescapable. We refer to this type of radiation as the background radiation. Cosmic rays originating from stars in space rain down upon the Earth. Radioactive atoms in the rocks and soil beneath our feet decay. Radon gas is in the air we breathe. This steady drizzle of radiation is part of the background. It’s not something you can turn on or off. It is the environmental baseline. If there was no technology, no nuclear power plants, and no Chernobyl or Fukushima, there would still be a background radiation. It accounts for approximately 3 millisieverts (mSv) of exposure per year.
As we stated earlier, radiation is all around us at every moment of every day no matter where we are. Radiation is natural and inescapable. We refer to this type of radiation as the background radiation. Cosmic rays originating from stars in space rain down upon the Earth. Radioactive atoms in the rocks and soil beneath our feet decay. Radon gas is in the air we breathe. This steady drizzle of radiation is part of the background. It’s not something you can turn on or off. It is the environmental baseline. If there was no technology, no nuclear power plants, and no Chernobyl or Fukushima, there would still be a background radiation. It accounts for approximately 3 millisieverts (mSv) of exposure per year.
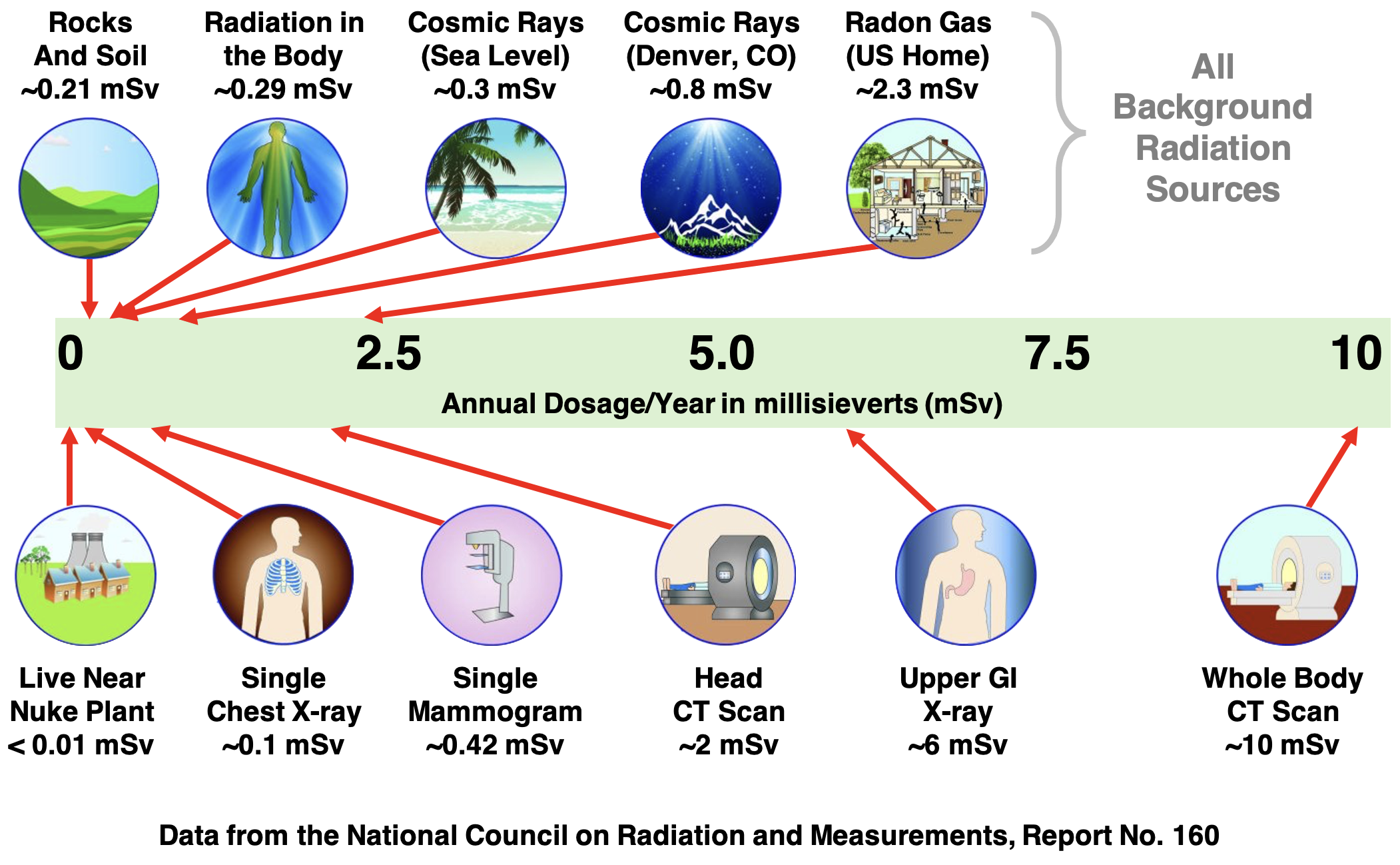
Image adapted from the Environmental Protection Agency
Human Contributions to Radiation
In addition to the natural sources of radiation, there are a variety of human contributions to the annual dose. Some of these are identified in the graphic above. As you inspect the graphic, keep two things in mind. First, the natural background radiation is approximately 3 millisieverts. Second, none of the values listed on the chart are anywhere near the levels that would cause health concerns. A single chest X-ray could save your life ... depending on what it might uncover. On the other hand, 18,000 chest X-rays in the course of a year will put your life at great risk. (So, go easy on those chest X-rays.)
There are a few things worth noting regarding human contributions to radiation exposure:
- It is usually a surprise to most students that living near a nuclear power plant contributes very little to the annual dosage that a person receives. Eating one banana would contribute as much or more to one’s exposure.
- The atmosphere provides some shielding from cosmic rays. A thicker atmosphere means more shielding. An airplane flight across the country and living in a high-altitude location would increase your annual dosage since the atmosphere is thinner for higher altitudes.
- Much of the extra dosage above the background radiation levels can be attributed to medical procedures that have very good purpose. Do the procedure if prescribed! Medical professionals know that they contribute to your radiation exposure. The procedure is prescribed in light of that knowledge ... not in disregard for it.
- The United States began above-ground nuclear weapons testing in 1945. Other countries such as the United Kingdom, the Soviet Union, and France did the same in the 1950s and 1960s. In 1963, the US, UK, and USSR signed a treaty to cease above-ground testing. Above ground nuclear weapons testing continued up until 1980 by the People’s Republic of China. Unfortunately, the tests released radioactive fallout that had a global spread. This period of time marks the largest deliberate release of radioactive material into the environment. Two radioisotopes in particular - Strontium-90 and Cesium-137 - have half-lives on the order of 30 years. They are still present in our environment today.

Would you like to do an estimate of your personal radiation dose? Use the
Nuclear Regulatory Commission’s dose worksheet to calculate your estimated annual dose in millirems. Then divide by 100 to convert to milliSievert (mSv).
Radiation Doses and Health Effects
So far ... nothing bad. Now it’s time to be bad. Then on the next page, we’ll get downright ugly. But before we get ugly, let’s address the proverbial elephant in the room question. At what point does one’s exposure to radiation cause a health concern? How much radiation will kill a person? Should I throw my smoke detector out because it contains radioactive americium-241? Should I stop eating bananas? Should I move from Colorado to New Orleans to insure more protection from cosmic rays?
 Ionizing radiation
Ionizing radiation possesses enough energy to break bonds between atoms in the cells of organisms. The health impact of
ionizing radiation depends upon the dose,
the type of radiation, and the duration. Not surprisingly higher doses increase the risk of harm and damage. The type also matters.
Alpha particles cause more damage if emitted from radioisotopes that are already in the body due to ingestion or inhalation.
Beta particles have less ionizing ability but can penetrate the body when emitted from external sources. Ultraviolet radiation (like the type that causes sunburns) is an ionizing form of radiation. But it is less harmful than the radiation from nuclear sources that we have been discussing in this chapter.

The body has the ability to repair itself. Those who have been burned by ultraviolet rays from the Sun are familiar with the body’s ability to repair itself. But if exposure to high levels of
ionizing radiation such as alpha, beta, and gamma radiation occur in a short amount of time (minutes, hours, days), the body’s ability to repair itself becomes overwhelmed and a negative health impact is almost certain. Such impacts can range from severe burns on the skin to death within a short period of time. So how is a person exposed to high levels in a short amount of time? This is most commonly associated with exposures to a nearby nuclear blast and the resulting nuclear fallout or with a massive leak of radiation from a nuclear power plant accident such as what occurred at Chernobyl. This is what we have been referring to as
The Ugly. We will discuss this type of exposure in more detail in
Lesson 4c.

Because the body can repair itself from damage if given enough time, it has become conventional to monitor the dosage of radiation on a per year basis. Certain occupations are at more risk of exposure than others. Governmental agencies set exposure limits for such workers and require that workers wear a film badge (dosimeter) to detect cumulative exposure levels. In the US, a worker in a nuclear industry cannot have an exposure exceeding 50 mSv/year.
Image Source:
Wikimedia Commons
Studies associating exposure levels with cancer risks do exist. Scientists generally assume that
any amount of
ionizing radiation might slightly raise the risk of developing cancer. This is called the
linear no-threshold (LNT) model. But at low doses, like 10 mSv/year, the increase is so small that it’s very hard to detect directly in human studies. Strong evidence of increased cancer risk clearly exists at high doses like 100 mSv/year, but at lower levels the effect is modest compared with many everyday risks. For example, smoking cigarettes has a much larger and well-documented impact on cancer risk than low-dose radiation. Lifestyle factors such as diet and smoking contribute far more to most people’s overall cancer risk than small increases in radiation exposure. An increased risk does
not mean someone will definitely get cancer, only that the probability is somewhat higher.
A careful study of the
Radiation Dose Chart below will help you put the topic of exposures and risks in perspective. The blue squares are microsievert amounts less than 50 µSv. The green squares are millisievert amounts less than or equal to 50 mSv. And the red squares extend from the bad to the
ugly side of radiation - greater than 50 mSv on up to 8 Sv.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Radiation Dose and Effects. Save it to a safe location and use it as a review tool.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Identify the following as being either natural background sources of radiation (NB) or as human contributions to radiation (HC).
- Cosmic rays lead to the production of carbon-14 which finds its way into our food.
- Americium-241 in a smoke detector emits an alpha particle.
- The doctor takes an x-ray of your arm to see if there is a fracture.
- You ingest several potassium-40 radionuclides while eating your banana split.
- Your exposed to a small amount of radiation from the bricks of your home.
- You are given a PET scan of your brain to determine how superhuman you are.
- Radioactive radon gas seeps into your home through the cracks in the foundation.
- You have acquired your grandmother’s FiestaWare dinner plates, not knowing that they were mildly radioactive.
For Questions #2-#7, identify the statements as being either TRUE or FALSE. If False, then either correct the statement or identify what is wrong with it.
2. TRUE or FALSE:
1 sievert is a very small amount of radiation. Thus, the megasievert (Ms) is more commonly used.
3.
TRUE or
FALSE:
Any exposure to radiation that exceeds the background radiation amount will result in cancer.
4.
TRUE or
FALSE:
A trip across the country on an airplane exposes you to more radiation because the plane is made from radioactive material.
5.
TRUE or
FALSE:
Living near a nuclear power plant exposes you to a large amount of radiation.
6.
TRUE or
FALSE:
Cell phones cause a good deal of radiation and are one of the causes of brain tumors.
7.
TRUE or
FALSE:
Alpha particles are the most dangerous type of radiation since they have the greatest penetration ability.
8.
Half-Life Review: Strontium-90 was released into the environment during above-ground nuclear weapons testing in the last century. The US halted the practice in 1963. If the Sr-90 has a half-life of 28 years, approximately what percentage of the 1963-amount remains in the environment today?