Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Solubility of Salts
Part c: Solubility and Common Ion Effects
Part a:
The Solubility Product Constant, Ksp
Part b:
Solubility and Ksp
Part c: Solubility and Common Ion Effects
The Big Idea
The page explains how solubility isn’t fixed. When an insoluble salt is dissolved in a solution that already contains one of its ions, the equilibrium system responds by reducing the solubility. Learn the concept and the mathematics in the usual organized presentation.
What is a Common Ion?
The solubility of slightly or sparingly soluble salts (sometimes referred to as insoluble salts) in pure water was discussed in Lesson 1b. The ion concentration was determined by assuming that the dissociating salt was the only source of the ions. This is the case for salts dissolving in pure water. An ICE table analysis indicates that the initial concentration of the ions was 0 M.
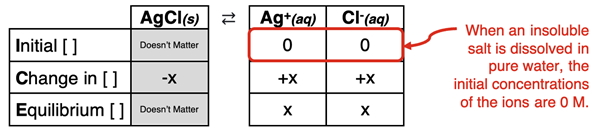
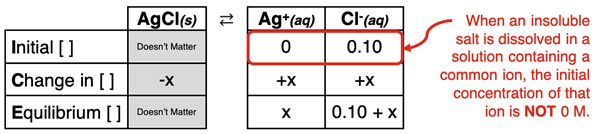
In this part of Lesson 1, we will discuss situations in which the insoluble salt is dissolved in a solution containing a common ion. This most often happens when the solution contains a soluble salt having an ion in common with the insoluble salt. For instance, if AgCl (an insoluble salt) is dissolved in a solution of 0.10 M NaCl (a soluble salt), the chloride ion is a common ion. It is present in both salts or common to both salts. As such, the chloride ion is already in the solution before the insoluble salt dissociates. Its presence will affect the solubility of the AgCl.
The beaker diagrams below represent situations in which an insoluble salt is being dissolved in a solution containing a common ion. A soluble salt that has an ion in common with the insoluble salt is present in the solution before the insoluble salt is added. Each of these situations leads to what we refer to as the common ion effect.
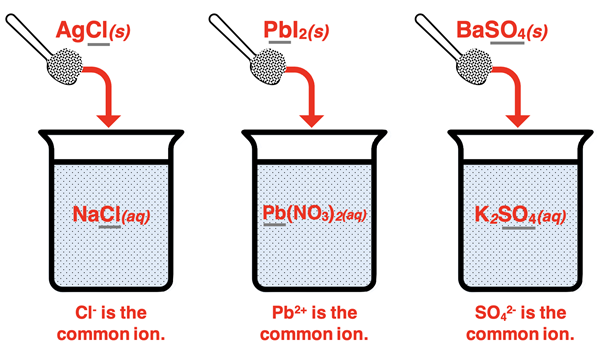
What is the Common Ion Effect?
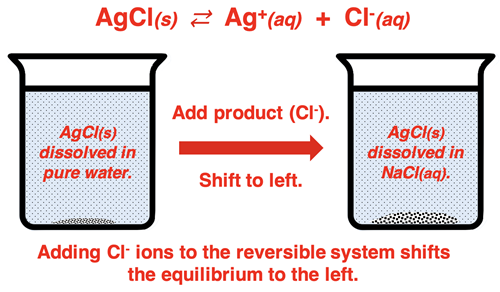
When an insoluble salt is dissolved in a solution containing a common ion, the solubility of the salt decreases. While the Ksp of the salt is unaffected by the common ion, the amount of salt that can dissociate before equilibrium is reached is significantly reduced. This decrease in solubility due to the presence of a common ion can be explained by LeChatelier’s principle. If you imagine the two ions in otherwise pure water in equilibrium with undissolved solid, the addition of one of the product ions disrupts the equilibrium and shift the reaction towards the left. This LeChatelier shift towards reactants reduces the amount of solid that is dissolving.
The following statements illustrate the common ion effect. Observe the significant decrease in solubility that occurs when the insoluble salt is dissolved in a solution of a soluble salt that contains a common ion.
- The solubility of AgCl in pure water is 1.3x10-5 mol/L. But its solubility in a 0.10 M NaCl solution is 1.8x10-9 mol/L.
- The solubility of CaF2 in pure water is 2.1x10-4 mol/L. But its solubility in a 0.10 M NaF solution is 3.5x10-9 mol/L.
- The solubility of AlPO4 in pure water is 9.9x10-11 mol/L. But its solubility in a 0.10 M K3PO4 solution is 9.8x10-20 mol/L.
A Simplifying Assumption
The mathematical analysis of the common ion effect can be algebraically complex. Fortunately, there is an assumption that is commonly made that simplifies the algebra. The assumption is that the concentration of the common ion in the solution is primarily due to the contribution made by the soluble salt. If the soluble salt dissociates to produce the common ion with a concentration of 0.10 M, then the dissolving of the insoluble salt contributes such a small amount to this concentration value that it will still be approximately 0.10 M. For instance, if x mole/L of the common ion is produced by the insoluble salt’s dissociation, then the equilibrium concentration would be 0.10 + x. For insoluble salts with very small K
sp values, x is generally so small that ...
0.10 + x ≈ 0.10.
Case Comparison - Solubility of AgCl With and Without a Common Ion
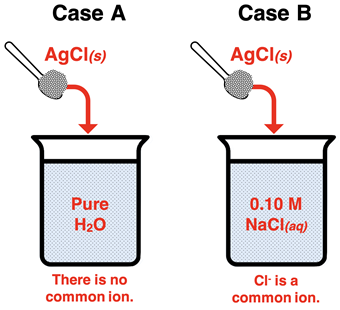
Let’s conduct a mathematical analysis comparing the solubility of AgCl in pure water (
Case A) to the solubility in a 0.10 M solution of NaCl (
Case B). In both cases, the AgCl is an ever-so-slightly soluble salt with a K
sp value of 1.8 × 10
-10. It dissociates to produce the Ag
+ and Cl
- ions:
AgCl(s) ⇄ Ag+(aq) + Cl-(aq)
When dissolved in pure water, there is no common ion effect. When dissolved in the 0.10 M NaCl(aq) solution, there is a common ion effect. The chloride ion is the common ion. Since NaCl is a soluble salt, the concentration of Cl
- is initially 0.10 M. We will account for this initial concentration in an ICE table. For both Case A and Case B, we can write the solubility product constant equation as:
Ksp = [Ag+] • [Cl-]
The ICE tables and subsequent analysis for the two cases are shown below.
The mathematics shows that the presence of the common ion (Cl
-) in Case B causes a noticeably lower solubility for the insoluble salt. This is the common ion effect!
Step-by-Step Procedure for Solving a Common Ion Problem
The above analysis depicts a procedural approach to the solution of a common ion problem for an insoluble salt dissolved in a solution of a soluble salt. The procedure can be described by the following steps.
- Write the balanced chemical equation for the dissociation of the insoluble salt. (Need help? Learn More.)
- Write the Ksp equation for the reaction. (Need help? Learn More.)
- Identify the common ion present in the soluble salt and use the concentration of the soluble salt to determine the concentration of the common ion in the solution.
- Set up an ICE table. Use the initial concentration of the common ion in the top row of the table. Use the variable x to represent the amount of solid that dissolves. Use the simplifying assumption to reduce the math complexity.
- Substitute the Ksp value and the expressions for the equilibrium concentrations of the ions into the Ksp equation from Step 2.
- Use algebra to solve for the x; the value of x is the solubility of the insoluble salt.
Example 1 - Dissolving CaF2 in a 0.10 M NaF Solution
Calcium fluoride is an insoluble salt with a K
sp value of 3.5 × 10
-11. Determine its solubility in a 0.10 M solution of NaF.
Solution
Step 1: The equation for the dissociation of CaF
2 is:
CaF2(s) ⇄ Ca2+(aq) + 2 F-(aq)
Step 2: The K
sp equation is:
Ksp = [Ca2+] • [F-]2
Step 3: The CaF
2 is being dissolved in a solution of NaF. This solution contains F
- ions; it is the common ion. The concentration of the F- in this solution is initially 0.10 M.
Step 4: The completed ICE table is shown below. Before dissociation, there is no Ca
2+ ion in the solution but the F
- ion is present with a concentration of 0.10 M; that’s the
Initial row. An unknown amount of ions are produced (“x” and “2x”); the coefficients in the balanced equation are used for this
Change row. The
Equilibrium row of an ICE table is always determined by adding the
Change row to the
Initial row. We wish to determine x. Since solids are not included in the equilibrium constant expression, we do not worry about their concentrations; that explains why the first column is greyed out.
Step 5: The K
sp value and the last row of the ICE table are substituted into the equation written in Step 2. Note how the simplifying assumption has been used for the chloride ion concentration: 0.10 + 2x ≈ 0.10.
3.5 × 10-11 = x • (0.10)2
Step 6: Algebra is used to solve for x. The value of x is the solubility. Perform the algebra as slowly as your comfort level requires; do not rush! Slowing down is often the fastest way to a successful finish.
3.5 × 10
-11 = x • (0.10)
2
3.5 × 10
-11 / (0.10)
2 = x
3.5 × 10
-9 = x
Solubility = 3.5 × 10-9 mol/L
Example 2 - Dissolving BaSO4 in a 0.10 M Na2SO4 Solution
Barium sulfate is an insoluble salt with a K
sp value of 1.1 × 10
-10. Determine its solubility in a 0.10 M solution of Na
2SO
4.
Solution
Step 1: The equation for the dissociation of BaSO
4 is:
BaSO4(s) ⇄ Ba2+(aq) + SO42-(aq)
Step 2: The K
sp equation is:
Ksp = [Ba2+] • [SO42-]
Step 3: The BaSO
4 is being dissolved in a solution of Na
2SO
4. This solution contains SO
42- ions; it is the common ion. The concentration of the SO
42- in this solution is initially 0.10 M.
Step 4: The completed ICE table is shown below. Before dissociation, there is no Ba
2+ ion in the solution but the SO
42- ion is present with a concentration of 0.10 M; that’s the
Initial row. An unknown amount of ions are produced (“x”); the coefficients in the balanced equation are used for this
Change row. The
Equilibrium row of an ICE table is always determined by adding the
Change row to the
Initial row. We wish to determine x. Since solids are not included in the equilibrium constant expression, we do not worry about their concentrations; that explains why the first column is greyed out.
Step 5: The K
sp value and the last row of the ICE table are substituted into the equation written in Step 2. Note how the simplifying assumption has been used for the chloride ion concentration: 0.10 + x ≈ 0.10.
1.1 × 10-10 = x • 0.10
Step 6: Algebra is used to solve for x. The value of x is the solubility.
1.1 × 10-10 = x • 0.10
1.1 × 10-10 / 0.10 = x
1.1 × 10-9 = x
Solubility = 1.1 × 10-9 mol/L
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Common Ion Effects for Insoluble Salts. Save it to a safe location and use it as a review tool.
Check Your Understanding of the Common Ion Effect
Use the following questions to assess your understanding of the common ion effect and your skill at solving common ion problems. Tap the Check Answer buttons when ready.
1. Identify the following statements as being TRUE or FALSE:
- The common ion is always an anion.
- The presence of a common ion will decrease the Ksp of the insoluble salt.
- The presence of a common ion can either increase or decrease the solubility, depending on whether it is positively or negatively charged.
- The effect that a common ion has upon solubility is not explainable. We know it changes the solubility but we don’t fully understand why.
- Common ion problems are not possible to solve without making a simplifying assumption.
- Common ion problems are best solved by assuming that the concentration of the common ion is the average of what is contributed by the soluble salt and the insoluble salt.
2. For the following situations, identify the symbol of the common ion and its initial molar concentration.
a. CaCO3 dissolving in a solution of 0.10 M CaCl2.
b. CaCO
3 dissolving in a solution of 0.10 M Na
2CO
3.
c. CuCl dissolving in a solution of 0.50 M CuNO
3.
d. Ca
3(PO
4)
2 dissolving in a solution of 0.50 M Ca(C
2H
3O
2)
2.
e. Ca
3(PO
4)
2 dissolving in a solution of 0.25 M Na
3PO
4.
3. Barium sulfate is an insoluble salt with a K
sp value of 1.1 × 10
-10. Determine its solubility in a 0.50 M solution of Ba(NO
3)
2.
4. Silver(I) carbonate is an insoluble salt with a K
sp value of 8.1 x 10
-12. Determine its solubility in a 0.50 M solution of AgNO
3.