Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Chemical Reactions and Enthalpy Change
Part d: Bond Enthalpy and ∆H
Part a:
Enthalpy Change
Part b:
Hess's Law
Part c:
Heat of Formation
Part d: Bond Enthalpy and ∆H
Part e:
Thermal Stoichiometry
The Big Idea
Estimating a reaction's enthalpy change (∆H) by using bond enthalpies helps us see why some reactions are exothermic and others are endothermic. By comparing the energy needed to break bonds to the energy released when new bonds form, we can estimate the net energy change and determine if a reaction is exothermic or endothermic.
Bond Breaking and Bond Forming
As we discussed in Lesson 1c of this Thermochemistry Chapter, chemical reactions can be thought of as occurring in two steps. First, bonds among reactant molecules are broken and atoms are pulled apart from one another. Second, atoms rearrange and new bonds are formed among product molecules. The first step - bond breaking - is an endothermic step; energy must be absorbed by the system to break the bonds. The second step - bond forming - is an exothermic step; energy is released by the system when bonds are formed. By comparing the energy required to break bonds within reactant molecules to the energy released when forming bonds within the product molecules, one can determine if the overall reaction is endothermic or exothermic.
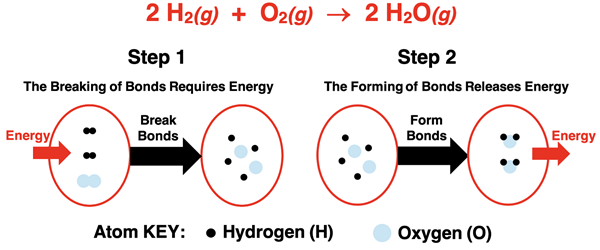
There are numerous ways to represent this stepwise model of a chemical reaction. In Lesson 1c, we used potential energy well diagrams to show the increase in system energy as reactant molecules are pulled apart and the decrease in system energy as product molecules are put together. You can review that discussion here.
 One of our favorite representations of this bond breaking and forming model is an enthalpy level diagram. The initial enthalpy of the system (reactant state) is shown on the diagram. As energy is absorbed by the system, the first step occurs. Bonds are broken and isolated atoms are formed at a higher enthalpy. The atoms rearrange and the second step of forming bonds occurs. The system lowers its enthalpy as energy is released to the surroundings.
One of our favorite representations of this bond breaking and forming model is an enthalpy level diagram. The initial enthalpy of the system (reactant state) is shown on the diagram. As energy is absorbed by the system, the first step occurs. Bonds are broken and isolated atoms are formed at a higher enthalpy. The atoms rearrange and the second step of forming bonds occurs. The system lowers its enthalpy as energy is released to the surroundings.
Whether this two-step process is overall endothermic or exothermic depends on how the energy requirements for breaking bonds compares to the energy release when forming bonds. If bond breaking requires more energy than released during bond formation, then there is a positive enthalpy change and an endothermic reaction. If bond formation releases more energy than is required for bond breaking, then there is a negative enthalpy change and an exothermic reaction (the case in the graphic above).
What is Bond Enthalpy?
One method of conducting the enthalpy comparison of this two-step process is to utilize bond enthalpy values for the bonds present in reactant and product molecules. The bond enthalpy is the amount of energy required to break 1 mole of a bond in a gaseous substance. Every bond has its own unique bond enthalpy value. The bond enthalpy of an H-O bond is different than the bond enthalpy of an H-H bond.
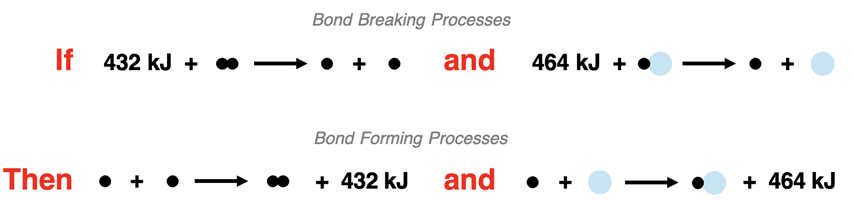
The bond enthalpy of an H-H bond is +432 kJ/mol. This value describes the energy required to break 1 mole of H-H bonds in the gas state. We can write this as a thermochemical equation and represent it as a particle diagram:
432 kJ + H-H(g) → H(g) + H(g)
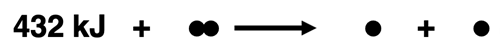
The bond enthalpy of an H-O bond is +464 kJ/mol. This value describes the energy required to break 1 mole of H-O bonds in the gas state. We can represent this with a thermochemical equation and a particle diagram:
464 kJ + H-O(g) → H(g) + O(g)
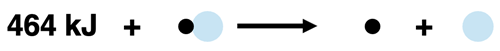
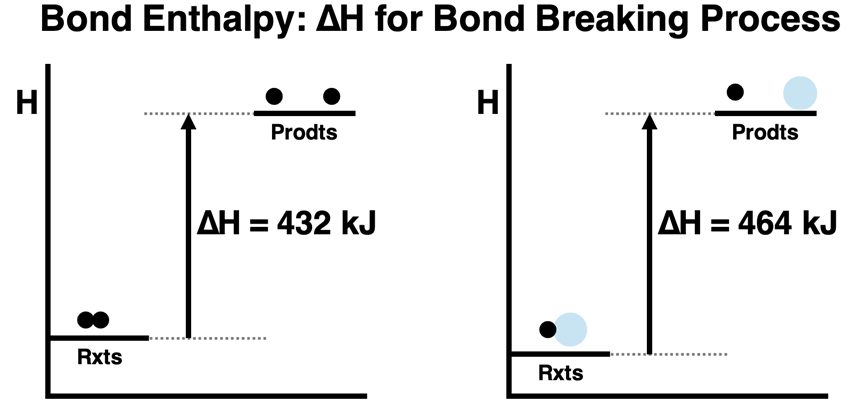
The energy released when forming a bond is equal to the energy required to break the same bond. The two processes – bond breaking and bond forming – are the reverse of each other. The ∆H values are equal in magnitude and have an opposite sign. The ∆H for forming 1 mole of H-H bonds is -432 kJ. And the ∆H for forming 1 mole of H-O bonds is -464 kJ.
It is worth mentioning that not every O-H bond is of equal strength. The O-H bonds of different substances do not have exactly the same bond enthalpy. For instance, it is expected that the O-H bonds in H2O differ slightly in strength than the O-H bonds in hydrogen peroxide (H2O2) and the O-H bond in methanol (CH3OH). The actual bond enthalpy values are determined by averaging the values for several different compounds. As such, a stated bond enthalpy value is a rough approximation of the enthalpy change for breaking that bond in a specific compound.
The table below lists bond enthalpy values for a variety of bonds.
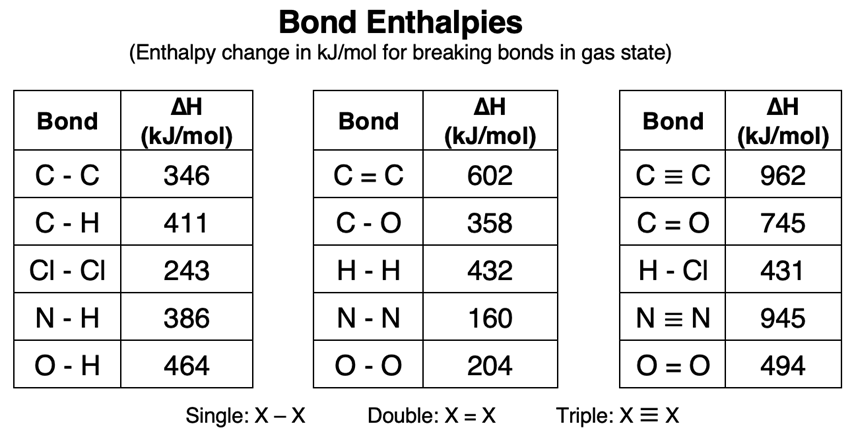
Bond Enthalpies for Double and Triple Bonds
In Chapter 6 of this Chemistry Tutorial, we learned about double bonds and triple bonds in molecular compounds. While a single bond involves the sharing of two electrons among covalently bonded atoms, a double bond involves the sharing of four electrons. And a triple bond involves the sharing of six electrons among covalently bonded atoms. An inspection of the top row of the table of Bond Enthalpies reveals that double bonded carbon atoms have a greater bond enthalpy than single bonded carbon atoms. And triple bonded carbon atoms have a greater bond enthalpy than double bonded carbon atoms. As more electrons are shared in a bond, the stronger that the bond is and the more energy that will be required to break it. The task of determining if a bond is a single, double, or triple bond requires that a Lewis electron dot diagram be constructed.
Conducting a Bond Inventory
Our goal in Lesson 2d is to be able to use bond enthalpy values to calculate an enthalpy change for a reaction. To accomplish this goal, we will need to develop the skill of inspecting the formulae for a chemical equation and determining what bonds and how many are being broken and formed. We will have to become skilled at conducting a bond inventory. Let’s do a few examples.
Our first bond inventory example will involve the synthesis of water vapor. The balanced chemical equation and the corresponding particle diagram are shown.
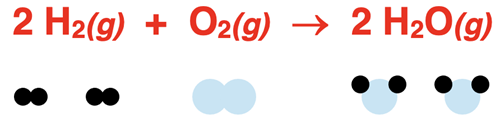
 On the reactant side, there are two H-H bonds – one in each H2 molecule. There is one O-O bond in the O2 molecule. If we think about the Lewis electron dot diagram for oxygen, we recognize that the O-O bond is a double bond. On the product side, there are four O-H bonds that must be formed – two in each water molecule.
On the reactant side, there are two H-H bonds – one in each H2 molecule. There is one O-O bond in the O2 molecule. If we think about the Lewis electron dot diagram for oxygen, we recognize that the O-O bond is a double bond. On the product side, there are four O-H bonds that must be formed – two in each water molecule.
Our bond inventory has identified the following bonds to be broken and formed:
- Break two H-H bonds
- Break one O=O double bond
- Form four H-O bonds
Our
second bond inventory example will be performed for the decomposition of phosphorus pentachloride into phosphorous trichloride and diatomic chlorine. The balanced chemical equation and the corresponding particle diagram are shown.
On the reactant side, there are five P-Cl bonds that must be broken. On the product side, there are three P-Cl bonds that must be formed and one Cl-Cl bond that must be formed. All bonds are single bonds.
Our bond inventory has identified the following bonds to be broken and formed:
- Break five P-Cl bonds
- Form three P-Cl bonds
- Form one Cl-Cl bonds
Our
third bond inventory example will be for the combustion of methane (CH
4) to form gaseous products of CO
2 and H
2O. The balanced chemical equation and the corresponding particle diagram are shown. We have also shown the Lewis electron dot diagram for O
2 and CO
2. Note that there are double bonds in each.
On the reactant side, there are four C-H bonds that must be broken. And there are two O=O double bonds that must be broken (one in each O
2 molecule). On the product side there are two C=O bonds that must be formed and there are four O-H bonds that must be formed (two in each H
2O molecule).
Our bond inventory has identified the following bonds to be broken and formed:
- Break four C-H bonds
- Break two O=O double bonds
- Form two C=O double bonds
- Form four H-O bonds
How to Use Bond Enthalpies to Calculate ∆H Values
Our model of chemical reactions as bond-breaking and bond-forming events allows us to use bond enthalpy values to calculate ∆H values for reactions. Consistent with
Hess’s Law, the ∆H of the reaction is the sum of all the bond breaking steps and bond forming steps. The task of calculating the enthalpy change for a reaction completed with this five-step procedure:
- Conduct a Bond Inventory for the Reaction
Determine the types of bonds being broken and formed. Determine the number of each type. A particle diagram may be a helpful tool. Use Lewis electron dot diagrams to determine if the bonds are single bonds, double bonds, or triple bonds.
- Look up Bond Enthalpy Values
For each bond being broken and formed, look up the bond enthalpy values in the table of Bond Enthalpies. These will be used to determine the ∆H of all bond breaking and bond forming steps.
- Calculate the ∆H for Bond Breaking
For any bond being broken, multiply the number of bonds being broken by the bond enthalpy. Sum all the values together to determine the ∆H of the bond breaking steps.
- Calculate the ∆H for Bond Forming
The ∆H for forming a bond is negative. It is simply the bond enthalpy value written with a negative sign. For any bond being formed, multiply the number of bonds being formed by the bond enthalpy; put a negative in front of the result. Sum all these negative values together to determine the ∆H of the bond forming steps.
- Determine the Overall Enthalpy Change
Add the ∆H for bond breaking (step 3, a positive number) to the ∆H for bond forming (step 4, a negative number). The result is the enthalpy change of the reaction.
If math equations are your preference, then you can use this one:
∆Hreaction = ∑ Bond Enthalpybonds-broken + (-1• ∑ Bond Enthalpybonds-formed)
Bond enthalpy values are based on 1 mole of bonds. If the bond inventory indicates that more than one bond of a given type is being broken or formed, then you will need to multiply its bond enthalpy by the number of bonds.
The examples below demonstrate this 5-step method.
Example 1 - Using Bond Enthlpies to Determine a ∆H for a Reaction
Use bond enthalpy values to determine the heat of reaction for the synthesis of water vapor from its elements:
2 H2(g) + O2(g) → 2 H2O(g)
Solution:
We conducted a bond inventory above and identified the following bonds to be broken and formed.
- Break two H-H bonds
- Break one O=O double bond
- Form four H-O bonds
The bond enthalpy values from the
table of Bond Enthalpies above are:
- H-H bond: +432 kJ/mol
- O=O double bond: +494 kJ/mol
- H-O bond: +464 kJ/mol
The bond breaking step will require the following amount of energy. This is the ∆H for bond breaking:
2 mol•(+432 kJ/mol) + 1 mol•(+494 kJ/mol) = +1358 kJ
The bond forming step will release the following amount of energy. This is the ∆H for bond forming:
4 mol•(-464 kJ/mol) = -1856 kJ
Now the enthalpy change for the reaction can be determined by adding the positive ∆H for bond breaking to the negative ∆H for bond forming.
∆H = +1358 kJ + (-1856 kJ)
∆H = -498 kJ
The reaction is exothermic and releases 498 kJ for the synthesis of two moles of water vapor.
Example 2 - Using Bond Enthlpies to Determine a ∆H for a Reaction
Use bond enthalpy values to determine the heat of reaction for the synthesis of ammonia (NH
3):
N2(g) + 3 H2(g) → 2 NH3(g)
Solution:
The particle diagram for this synthesis reaction is shown below. The Lewis electron dot diagram for N
2 is also shown; it contains a (rare) triple bond.
There is one N≡N triple bond and three H-H bonds that will have to be broken in the reactants. And there are six N-H bonds (three for each NH
3 molecule) that will be formed in the product. The bond inventory can be summarized as:
- Break one N≡N triple bond
- Break three H-H bonds
- Form six N-H bonds
The bond enthalpy values from
table of Bond Enthalpies above are:
- N≡N triple bond: +945 kJ/mol
- H-H bond: +432 kJ/mol
- N-H bond: +386 kJ/mol
The bond breaking step will require the following amount of energy. This is the ∆H for bond breaking:
1 mol•(+945 kJ/mol) + 3 mol•(+432 kJ/mol) = +2241 kJ
The bond forming step will release the following amount of energy. This is the ∆H for bond forming:
6 mol•(-386 kJ/mol) = -2316 kJ
Now the enthalpy change for the reaction can be determined by adding the positive ∆H for bond breaking to the negative ∆H for bond forming.
∆H = +2241 kJ + (-2316 kJ)
∆H = -75 kJ
The reaction is exothermic and releases 75 kJ for the synthesis of two moles of ammonia.
Example 3 - Using Bond Enthlpies to Determine a ∆H for a Reaction
Use bond enthalpy values to determine the heat of reaction for the combustion of methane:
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
Solution:
We conducted a bond inventory above and identified the following bonds to be broken and formed.
- Break four C-H bonds
- Break two O=O double bonds
- Form two C=O double bonds
- Form four H-O bonds
The bond enthalpy values from the
table of Bond Enthalpies above are:
- C-H bond: +411 kJ/mol
- O=O double bond: +494 kJ/mol
- C=O double bond +745 kJ/mol
- H-O bond: +464 kJ/mol
The bond breaking step will require the following amount of energy. This is the ∆H for bond breaking:
4 mol•(+411 kJ/mol) + 2 mol•(+494 kJ/mol) = +2632 kJ
The bond forming step will release the following amount of energy. This is the ∆H for bond forming:
2 mol•(-745 kJ/mol) + 4 mol•(-464 kJ/mol)= -3346 kJ
Now the enthalpy change for the reaction can be determined by adding the positive ∆H for bond breaking to the negative ∆H for bond forming.
∆H = +2632 kJ + (-3346 kJ)
∆H = -714 kJ
The combustion of one mole of methane is exothermic and releases 714 kJ.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Our Calculator Pad section is the go-to location to practice solving problems. You’ll find plenty of practice problems on our Thermal Chemistry page. Here’s a good follow-up: Problem Set TC12: Bond Energy and Enthalpy Change.
- There is a conceptual exercise in our Science Reasoning Center titled Bond Energy and Reactions. It will serve as a great follow-up to this Tutorial page.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Bond Enthalpy and ∆H. Save it to a safe location and use it as a review tool.
Check Your Understanding of Bond Enthalpy and ∆H
Use the following questions to assess your understanding the relationship between bond enthalpy values and the ∆H of a reaction. Tap the Check Answer buttons when ready.
1. Associate the following statements with bond breaking of bond forming.
- Products are lower in enthalpy than reactants.
- Is an endothermic process.
- Pulling atoms apart.
- The ∆H value is positive.
- Heat is released as a result of the process.
- Putting atoms together to form molecules.
- Energy + H-H → H + H
- Is an exothermic process.
2. Use the
table of Bond Enthalpies to identify the ∆H of the following processes. Show the appropriate + or - sign.
- O-O → O + O ∆H = ???
- N + N → N-N ∆H = ???
- O + O → O=O (double bond) ∆H = ???
- C≡C (triple bond) → C + C ∆H = ???
3. The following is known about a particular reaction:
The bond enthalpies for all the reactant molecules is 1200 kJ. The bond enthalpies for all product molecules is 900 kJ. Which enthalpy level diagram is consistent with this information?
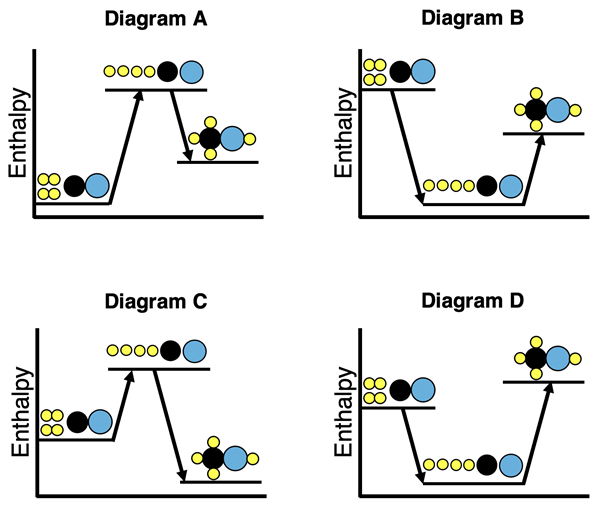
4. Use the
table of Bond Enthalpies above to determine the enthalpy change for the synthesis of hydrogen chloride (HCl) gas from its elements. Begin by writing the balanced chemical equation; then proceed with the 5-step procedure modeled in Examples 1 - 3.
5. Use the
table of Bond Enthalpies above to determine the enthalpy change for the combustion of propane (C
3H
8) gas to form gaseous products - CO
2 and H
2O. Begin by writing the balanced chemical equation; then proceed with the 5-step procedure modeled in Examples 1 - 3.