Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: What are Acids and Bases?
Part b: Models of Acids and Bases
Part a:
Properties of Acids and Bases
Part b: Models of Acids and Bases
The Big Idea:
Explore fundamental chemistry models, like the Arrhenius and Brønsted–Lowry models, to understand how acids and bases are defined by ionization or proton transfer tendencies when in aqueous solutions.
What is a Model in Chemistry?
Models are a big item in a Chemistry course. Nearly every topic has one or more models that must be learned to be successful. On this page we will be discussing two common models used to answer the question What are acids and bases? But before we discuss the models, let’s review what a model is.
 A model is a representation of a phenomenon or system that seeks to explain the relationship between its parts, its innerworkings, and its behavior. A model often includes diagrams, particle representations, charts, graphs, or mathematical equations. Models provide a mental framework around which students can organize ideas about the phenomenon or system. Models provide a common language and way of thinking about the topic, thus facilitating discussion and explanations. Models also help students to make predictions about how the object or system will behave under specified conditions.
A model is a representation of a phenomenon or system that seeks to explain the relationship between its parts, its innerworkings, and its behavior. A model often includes diagrams, particle representations, charts, graphs, or mathematical equations. Models provide a mental framework around which students can organize ideas about the phenomenon or system. Models provide a common language and way of thinking about the topic, thus facilitating discussion and explanations. Models also help students to make predictions about how the object or system will behave under specified conditions.
We have encountered numerous models in this Chemistry Tutorial. Some of the bigger models include the quantum mechanical model of the atom, the valence shell electron pair repulsion model of molecular geometry, and the kinetic-molecular model of ideal gas behavior. In this chapter, we will develop models regarding acids and bases in aqueous solutions. Let’s begin with our first model.
The Arrhenius Model of Acids and Bases
Our first model of acids and bases was proposed by Swedish chemist Svante Arrhenius in 1884. The Arrhenius model proposes that an acid is a compound that produces H+ ions when dissolved in water. The Arrehenius model identifies compounds like HCl, HBr, HNO3, H2SO4, and HClO4 as examples of acids. When any of these dissolve in water, they will dissociate and the concentration of H+ ions in solution will increase. The process is represented by acid dissociation equations that show H+ ions as a product:
HCl(aq) → H+(aq) + Cl-(aq)
HBr(aq) → H+(aq) + Br-(aq)
HNO3(aq) → H+(aq) + NO3-(aq)
According to the Arrhenius model, an acid has the generic formula HA where A is an anion like Cl-, Br-, NO3-, etc. The dissociation of the acid in water releases the H+ ion, giving the substance its acidic nature.
An Arrhenius base is a a compound that produces OH- ions when dissolved in water. The Arrehenius model would identify compounds like NaOH, KOH, LiOH, Ca(OH)2, and Sr(OH)2 as examples of bases. When any of these dissolve in water, they will dissociate and the concentration of OH- ions in solution will increase. The base dissociation equations explain why a base increases the hydroxide ion concentration in the solution.
NaOH(aq) → Na+(aq) + OH-(aq)
KOH(aq) → K+(aq) + OH-(aq)
Ca(OH)2(aq) → Ca2+(aq) + 2 OH-(aq)
The Arrhenius model predicts that a base would have the generic formula MOH where M is a cation. Its dissociation in water releases the OH- ion, giving the substance its alkaline nature.
The Brønsted-Lowry Model of Acids and Bases
The Arrhenius model defines acids and bases as containing H+ ions and OH- ions. While this 1884-model provided valuable insight into acids and bases, its obvious shortcoming was its inability to explain why substances that do not contain H+ or OH- ions exhibit acidic or basic behavior. For instance, ammonia (NH3) is a base despite the fact that it does not contain OH- ions. A second model proposed in 1923 by Johannes Brønsted and Thomas Lowry provided an expanded definition of acids and bases that addressed many of the shortcomings of the Arrhenius model.
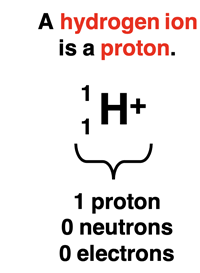
The Brønsted-Lowry model defines an acid as a hydrogen ion donor and a base as a hydrogen ion acceptor. Let’s pause here and think a bit deeper about what a hydrogen ion is. A hydrogen atom with an atomic number of 1 and a mass number of 1 (the most common isotope) contains 1 proton, 0 neutrons, and 1 electron. When the H atom forms a positive ion, it loses the electron, leaving the H atom with a single proton. A hydrogen ion is a proton. So, another way to express the Brønsted-Lowry definition of an acid and a base is to define an acid as a proton donor and a base as a proton acceptor.

The Brønsted-Lowry model shifts the focus from what a substance is to how it behaves. While Arrhenius asserted acids contain H+ and bases contain OH-, the Brønsted-Lowry model defines acids and bases by their role in proton transfer reactions. Acids donate protons and bases accept them. As we will soon see, it is possible for the same substance to behave as an acid under certain conditions and as a base under other conditions. Being able to identify a substance as an acid or a base requires being able to observe whether it is donating or accepting a proton (i.e., H+ ion) in a chemical reaction.
Brønsted-Lowry Proton Transfer Reactions
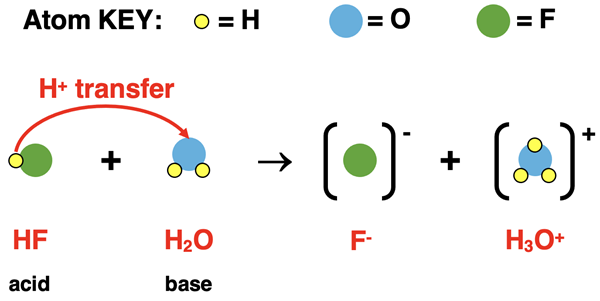
When a substance is behaving as an acid, there must be a second substance behaving as a base. To have a proton acceptor, there must be a proton donor. Hydrofluoric acid, HF, behaves as an acid in aqueous solution. When dissolved in pure water, it donates an H+ ion (proton) to water. The reactants are HF and H2O. After donating an H+ ion, the HF becomes F-. The H2O is acting as a base in this reaction. After accepting the H+ ion, the H2O becomes H3O+. The equation is written as
HF(aq) + H2O(l) → F-(aq) + H3O+(aq)
A particle diagram representation of this acid dissociation process is shown below. The H+ ion of the acid HF is shown being transferred to the H2O.
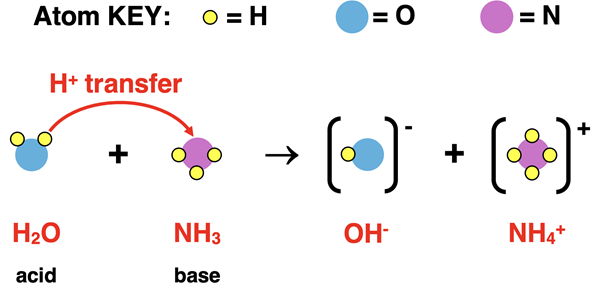
As a second example, let’s consider what happens when ammonia, NH3, is dissolved in water. NH3 is a base and will accept a proton. But it needs an acid to donate one. So now H2O will be serving as the acid. A proton (H+ ion) is transferred from the H2O to the NH3. This means that the H2O will turn into OH- since it has donated an H+ ion. And NH3 will turn into NH4+ since it has accepted the H+ ion. The equation is written as
NH3(aq) + H2O(l) → NH4+(aq) + OH-(aq)
A particle diagram representation of this base dissociation process is shown below. The H+ ion of H2O is shown being transferred to the NH3.
If given the reactant formulae and told which substance is the acid and/or which is the base, you will need to be able to write the proton transfer equation. Think in terms of an H+ ion being moved from the acid onto the base. Then identify the formula for what the acid becomes after losing (i.e., donating) an H+ ion. And identify the formula for what the base becomes after adding (i.e., accepting) an H+ ion. Use the next two examples to practice the skill. Once you have written the equation, tap the Check Answer button to check your answer and view the explanation.
Example 1 - Practice Writing Proton Transfer Equations
Write the proton transfer equation for the reaction between hydrocyanic acid (HCN) and water.
Example 2 - Practice Writing Proton Transfer Equations
Pyridine, C5H5N, is a base. Write the proton transfer equation for its reaction with H2O.
Conjugate Acid-Base Pairs
 Brønsted-Lowry acid-base equations consist of two pairs of similar particles (ions or molecules). These are referred to as conjugate acid-base pairs. There are two such pairs ... meaning four particles in all. In general, the word conjugate means a pair of joined-together items having similar forms. Two items that are described as being conjugate are not only similar in form but also differ from one another because of a small change or transformation. In acid-base chemistry, the two items in the pair are different than one another because of a proton (H+) transfer. The items in a conjugate acid-base pair differ from one another by one H+ ion.
Brønsted-Lowry acid-base equations consist of two pairs of similar particles (ions or molecules). These are referred to as conjugate acid-base pairs. There are two such pairs ... meaning four particles in all. In general, the word conjugate means a pair of joined-together items having similar forms. Two items that are described as being conjugate are not only similar in form but also differ from one another because of a small change or transformation. In acid-base chemistry, the two items in the pair are different than one another because of a proton (H+) transfer. The items in a conjugate acid-base pair differ from one another by one H+ ion.
The reactions we have studied so far are re-written below and the two pairs of conjugates are identified. Study the diagram and assure yourself that the definition of a conjugate acid-base pair makes sense.
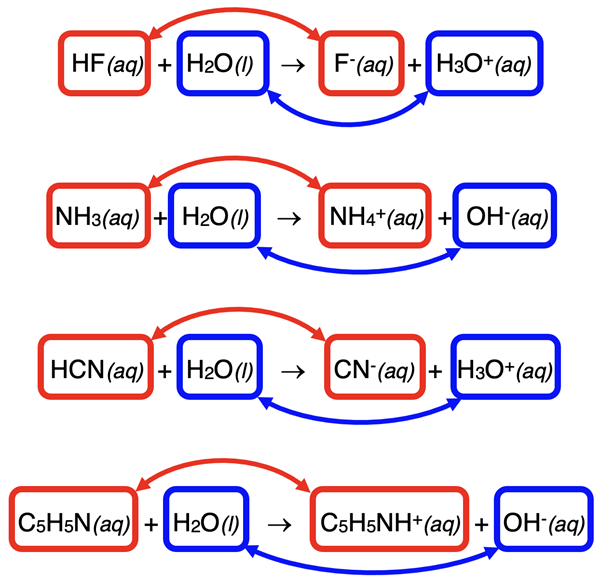
There’s more to the terminology. Each conjugate pair consists of an acid and a base. One of the pairs is called the acid and its conjugate base. The other pair is called the base and its conjugate acid. We have already spent considerable time identifying the acid and the base. The acids are the reactants that are donating a proton. The conjugate base is a product; it is what the acid turns into after losing the proton. Similarly, the base is the reactant that is accepting a proton. The conjugate acid is what the product that is formed from the base after it gains the proton.
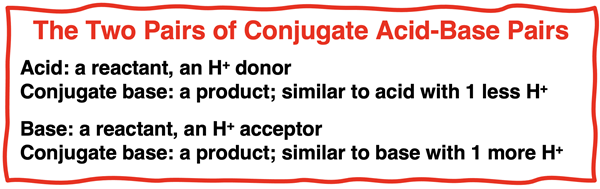
We have redone the above graphic, labeling the acid and its conjugate base for one pair and the base and its conjugate acid for the other pair. Once again, study the diagram and assure yourself that you are able to analyze an equation and determine the acid and its conjugate base and the base and its conjugate acid.
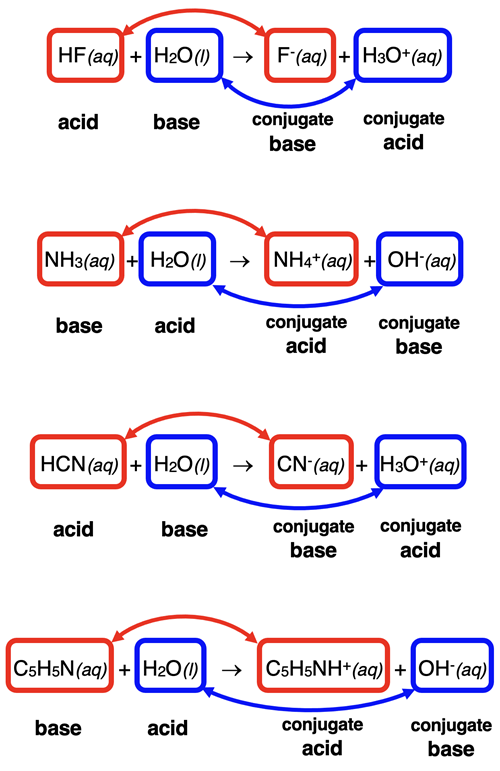
We can represent a Brønsted-Lowry acid-base equation with generic formulae as follows:
HA + B → A- + HB+
where HA is the acid reactant and B is the base reactant. On the product side, A- is the conjugate base of the acid and HB+ is the conjugate acid of the base. HA and A- are the acid-conjugate base pair. B and HB+ are the base-conjugate acid pair. The cartoon below further illustrates this concept.
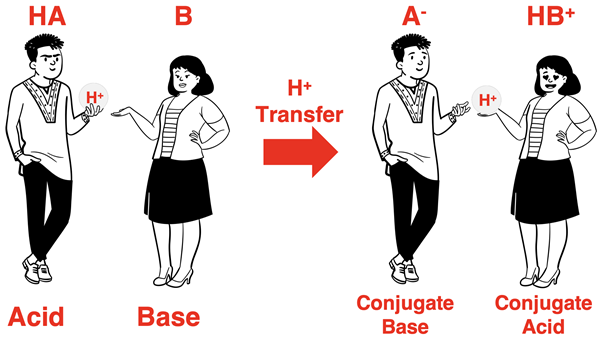
NOTE: Using generic formulae can be dangerous. There is no guarantee that the acid reactant will be neutral and its conjugate base has the - charge. It can only be guaranteed that they will differ by an H+ ion with the extra H+ being present on the acid reactant. A similar warning could be issued for B and HB+.
Example 3 - Practice with Conjugate Acid-Base Pairs
Suppose the following Brønsted-Lowry acid-base equations are written. For each, identify:
- the acid and its conjugate base
- the base and its conjugate acid
- HNO2(aq) + H2O(l) → NO2-(aq) + H3O+(aq)
- C2H5NH2(aq) + H2O(l) → C2H5NH3+(aq) + OH-(aq)
- HSO4-(aq) + CN-(aq) → SO42-(aq) + HCN(aq)
 Amphiprotic Substances
Amphiprotic Substances
There are a select number of substances that can behave as either an acid or a base depending upon the conditions. Such substances are referred to as being
amphiprotic. Water is a great example of an amphiprotic substance. Water behaves as a proton acceptor when it is in solution with an acid; and it behaves as a proton donor when it is in solution with a base.
Other amphiprotic substances include HSO
4-, HCO
3-, H
2PO
4-, and HPO
42-. For instance, the bicarbonate ion (HCO
3-) behaves as an acid in the presence of NH
3 (a base) and as a base in the presence of HF (an acid).
HCO3-(aq) + NH3(aq) → NH4+(aq) + CO32-(aq)
HCO3-(aq) + HF(aq) → F-(aq) + H2CO3(aq)
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Try our Concept Builder titled Brønsted-Lowry Model of Acids and Bases. Any one of the three activities provides a great follow-up to this lesson.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Models of Acids and Bases. Save it to a safe location and use it as a review tool.
Check Your Understanding of the Arrhenius and Brønsted-Lowry Models
Use the following questions to assess your understanding of the two acid-base models discussed on tis page. Tap the Check Answer buttons when ready.
1. Write the dissociation reactions for the following Arrhenius acids and bases:
a. LiOH
b. HClO
4
c. Ba(OH)
2
2. Write the Brønsted-Lowry acid-base equations for the following situations.
a. HCl is an acid that reacts with H
2O.
b. NO
2- is a base that reacts with H
2O.
c. HCl is an acid that reacts with NH
3.
d. HC
2H
3O
2 is an acid that reacts with H
2O.
e. C
2H
3O
2- is a base that reacts with H
2O.
f. HC
2H
3O
2 is an acid that reacts with CO
32-.
3. Write the Brønsted-Lowry acid-base equations for ...
a. HCO
3- acting as an acid in its reaction with H
2O.
b. HCO
3- acting as a base in its reaction with HC
2H
3O
2.
c. HCO
3- acting as an acid in its reaction with CN
-.
4. Identify the formula of ...
a. the conjugate acid of H
2O.
b. the conjugate base of H
2O.
c. the conjugate acid of NH
3.
d. the conjugate acid of OH
-.
e. the conjugate base of H
3PO
4.
f. the conjugate base of HPO
42-.
g. the conjugate acid of HPO
42-.
5. For the given Brønsted-Lowry acid-base equations, identify the acid, the base, the conjugate acid, and the conjugate base.
a. HCl
(aq) + H
2O
(l) → Cl
-(aq) + H
3O
+(aq)
b. HClO
4(aq) + H
2O
(l) → ClO
4-(aq) + H
3O
+(aq)
c. (CH
3)
3N + H
2O
(l) → (CH
3)
3NH
+(aq) + OH
-(aq)