Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Galvanic Cells
Part b: Reduction Potentials
Part a:
What is a Galvanic Cell?
Part b: Reduction Potentials
Part c:
Cell Voltage
Part d:
Batteries and Commercial Cells
The Big Idea
Reduction potentials reveal how strongly a substance attracts electrons. By comparing these values, we can predict which substances get reduced, which get oxidized, and the direction of electron flow in any electrochemical cell.
Electric Potential
 A galvanic cell can be described by an electric potential. The electric potential, also known as a voltage, provides a measure of the ability of the cell to produce a current. The higher the electric potential, the more current that the cell can produce. The electric potential of a galvanic cell is expressed in the unit volt.
A galvanic cell can be described by an electric potential. The electric potential, also known as a voltage, provides a measure of the ability of the cell to produce a current. The higher the electric potential, the more current that the cell can produce. The electric potential of a galvanic cell is expressed in the unit volt.
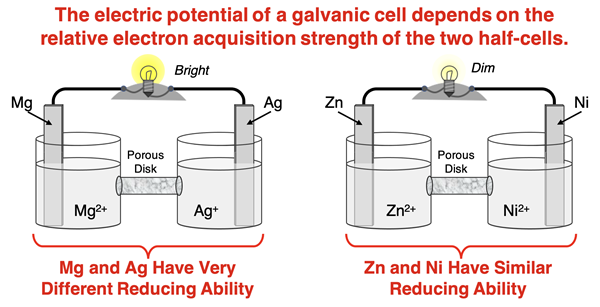
The electric potential of a galvanic cell depends upon the half-reactions that occur in the two half-cells. Think of the two half-cells as competing for the electrons. The cell which has the greatest tendency to acquire electrons is the one in which reduction will occur; it is the cathode. The other cell is the anode; oxidation occurs at that location. Electrons are lost at the anode where oxidation occurs and move through a wire to the cathode where reduction occurs. The rate at which the electrons flow through the wire is related to the current and is influenced by how differently the two half cells are in terms of their tendency to acquire electrons. Two half-cells with large differences in electron acquisition strength have a large electric potential and produce a large current.
Standard Reduction Potential
 The tendency of a half-cell to acquire electrons is expressed quantitatively as a standard reduction potential (Ered°). Every half-cell has its own unique standard reduction potential. It is not possible to directly measure the reduction potential of a single half-cell. So the strategy that has been universally adopted is to put the half-cell together with the standard hydrogen electrode (SHE) and to measure the overall cell voltage. The standard hydrogen electrode refers to a half cell with an aqueous solution of 1.00 M H+, hydrogen gas at 1.00 atm pressure, a temperature of 298 K, and a platinum (Pt) electrode. By convention, such a cell is assigned a reduction potential of 0.00 V.
The tendency of a half-cell to acquire electrons is expressed quantitatively as a standard reduction potential (Ered°). Every half-cell has its own unique standard reduction potential. It is not possible to directly measure the reduction potential of a single half-cell. So the strategy that has been universally adopted is to put the half-cell together with the standard hydrogen electrode (SHE) and to measure the overall cell voltage. The standard hydrogen electrode refers to a half cell with an aqueous solution of 1.00 M H+, hydrogen gas at 1.00 atm pressure, a temperature of 298 K, and a platinum (Pt) electrode. By convention, such a cell is assigned a reduction potential of 0.00 V.
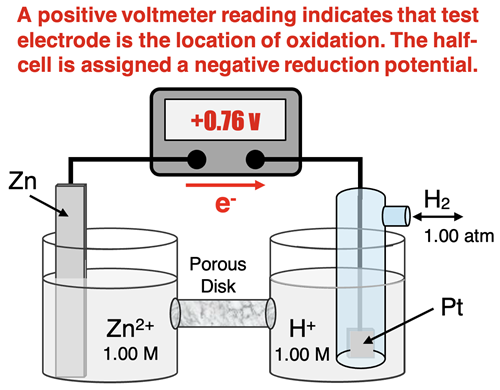
When determining the reduction potential of a half-cell, it is connected to the H+ | H2 half cell. A voltmeter is used to measure the cell voltage. The test electrode is connected to the positve terminal (red) of the voltmeter and the SHE is connected to the negative terminal (black) of the voltmeter. A reading is obtained and the reduction potential of the half-cell is determined relative to the standard hydrogen electrode (SHE).
Now here is where the discussion gets tricky. A voltmeter provides a sign (+ or -) and a value. The sign is an indicator of which direction the electrons are moving, and thus indicate which is the anode in the set-up. When set up as described in the previous paragraph, a positive voltmeter reading indicates that the test electrode is the anode and the SHE is the cathode. So a positive voltmeter reading indicates that the test half-cell has a lower electron acquistion ability relative to standard hydrogen electrode (SHE). Rather than being the site of reduction, it is the site of oxidation. Because of this, we assign the half-cell a negative reduction potential value. This negative reduction potential value indicates that it is less likely to undergo reduction than the standard hydrogen electrode.
As an example, consider the Zn | Zn2+ half cell. The tendency for Zn2+ ions to be reduced to Zn is relatively weak when compared to the tendency of H+ ions to the reduced to H2. So oxidation occurs at the zinc electrode and reduction occurs at the standard hydrogen electrode. Electrons move from the zinc to the SHE and a positive voltage is observed. Yet, the Zn | Zn2+ half cell is assigned a reduction potential value of -0.76 volts. This negative value indicates that the Zn | Zn2+ half cell is less likely than the hydrogen half cell to be reduced.
As a second example, consider the Ag |Ag+ half cell. The tendency for Ag+ ions to be reduced to Ag is relatively strong when compared to the tendency of H+ ions to the reduced to H2. So reduction occurs at the silver electrode and oxidation occurs at the standard hydrogen electrode. Electrons move from the SHE to the silver and a negative voltage is observe. Yet, the Zn | Zn2+ half cell is assigned a reduction potential value of +0.80 volts. This positive value indicates that reduction is more likely to occur in the the Ag | Ag+ half cell than in the hydrogen half cell.
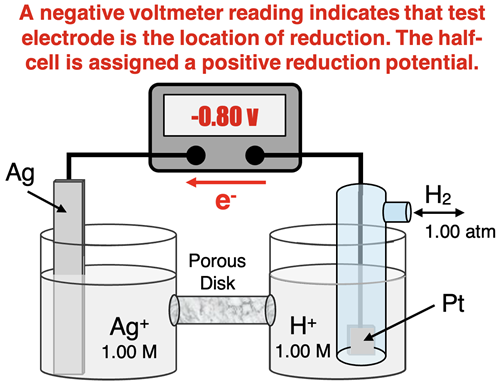
These two examples lead to the following conclusions regarding reduction potentials.
- A negative reduction potential value is an indicator of a weak tendency to be reduced (relative to the SHE).
- The more negative the value is, the less likely that reduction will occur.
- A positive reduction potential value is an indicator of a strong tendency to be reduced (relative to the SHE).
- The more positive the value is, the more likely that reduction will occur.
The standard reduction potentials of all other half-cells are measured in the same manner relative to the SHE. The word
standard of standard reduction potentials infers that all solutes have a concentration of 1.00 M, all gases have a pressure of 1.00 atm, and the temperature is 298 K. As we will learn in Lesson 2c, the voltage reading will be affected by the concentration, pressure, and temperature.
The table below shows standard reduction potentials for a select number of half cells. A
lengthier list of reduction potentials is available in the
Reference section of our
Chemistry Tutorial.
Strength of Oxidizing Agents and Reducing Agents
The table above lists
reduction potentials (E° or
Ered). If the half-equation is reversed so that it become an oxidation half-equation, then the sign on the potential would have to be reversed and we would refer to it as an oxidation potential (
Eox). As an example, we could represent the Li | Li
+ half-cell in the following two ways.
Reduction: Li
+(aq) + e
- → Li
(s) E
red = -3.04 V
Oxidation: Li
(s) → Li
+(aq) + e
- E
ox = +3.04 V
As discussed in Lesson 1a, an
oxidizing agent is the reactant that is reduced. A
reducing agent is the reactant that is oxidized. The best oxidizing agents are those that are most readily reduced. They have the most positive reduction potential values. In the table above, F
2 is the strongest oxidizing agent of those that are listed. This is not surprising, since it is the element with the highest electronegativity.
In the standard reduction potential table, the
oxidizing agents are listed on the table to the left of the reaction symbol (→). The more positive the reduction potential (E°), the stronger that the oxidizing agent is. In contrast, the
reducing agents are listed on the table to the right of the reaction symbol (→). They undergo oxidation; the oxidation half-equation is the reverse of how it is written on the table. The strongest reducing agents have the most negative reduction potential values. For our table, this places them at the top of the table on the right.
This discussion of the relative strength of oxidizing and reducing agents allows us to predict the reaction that would spontaneously occur when any two half-cells are connected. We can predict that any oxidizing agent would react spontaneously with a reducing agent that is above it in the table (i.e., the product of a half-equation with a less positive E° value). For example, Cu
2+(aq) and Zn
(s) would react spontaneously; but Cu
(s) and Zn
2+(aq) would not react spontaneously.
As a second example, consider the half-reactions involving Ag and Ni (and their ions). The silver ion (Ag
+) is a stronger oxidizing agent than the nickel ion (Ni
+) since it has a more positive reduction potential. Similarly, Ni is a stronger reducing agent than Ag since its oxidation potential would be more positive than that of Ag. When these two half-cells are put together, the spontaneous reaction that occurs is the reaction of silver ions with nickel metal.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Try our Concept Builder titled Reduction Potential. Any one of the three activities provides a great follow-up to this lesson.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Reduction Potentials. Save it to a safe location and use it as a review tool.
Check Your Understanding of Reduction Potentials
Use the following questions to assess your understanding of how to interpret a reduction potential table. Tap the Check Answer buttons when ready.
1. A galvanic cell will have the greatest electric potential if the two half-cells _____.
- have very similar reduction potentials.
- both have positive reduction potentials
- both have negative reduction potentials
- have very different reduction potentials
2. “SHE” stands for ______.
- Spontaneous half-equation
- Student-hating electrons
- Standard hydrogen electrode
- Sulfur heliumide
3.
TRUE or
FALSE:
It is possible to directly measure the reduction potential of a half-cell without connecting it to another half-cell.
4. The strongest oxidizing agents are those that have ______.
- the most negative reduction potentials
- the most positive reduction potentials
- reduction potentials that are closest to 0 V
5. Use
the table of reduction potentials to determine which of the following would be the most effective reducing agent of Zn
2+.
- Cu2+
- Cu
- Na+
- Na
6. Use
the table of reduction potentials to determine which of the following would be the most effective oxidizing agent of Cu.
- Cl-
- Cl2
- Mg2+
- Mg
7. Use
the table of reduction potentials to determine which of the following reactions would occur spontaneously. Select all that apply.
- 2 Ag+(aq) + Cu(s) → 2 Ag(s) + Cu2+(aq)
- Zn2+(aq) + Ni(s) → Zn(s) + Ni2+(aq)
- Fe2+(aq) + Mg(s) → Fe(s) + Mg2+(aq)
- Na+(aq) + Ag(s) → Na(s) + Ag+(aq)