Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Spontaneity and Gibbs Free Energy
Part a: Predicting Spontaneity with Gibbs Free Energy
Part a: Predicting Spontaneity with Gibbs Free Energy
Part b:
Gibbs Free Energy and Equilibrium
The Big Idea
The Gibbs free energy equation gives us a single numerical indicator (ΔG) for predicting whether a reaction at constant pressure and temperature can proceed spontaneously. Learn how to use enthalpy change (ΔH) and the T•ΔS term to determine the spontaneity of a reaction.
Understanding Gibbs Free Energy
 The second law of thermodynamics associates spontaneous change with increasing entropy in the universe. This means two driving forces must be considered when predicting the spontaneity of any process - the ∆Ssystem and the ∆Ssurroundings. One driving force is a property of the surroundings and the other is a property of the system. Both driving forces must be considered. In some cases, the process is favored by one driving force and not favored by the other. In such cases, a temperature determines whether the reaction is spontaneous.
The second law of thermodynamics associates spontaneous change with increasing entropy in the universe. This means two driving forces must be considered when predicting the spontaneity of any process - the ∆Ssystem and the ∆Ssurroundings. One driving force is a property of the surroundings and the other is a property of the system. Both driving forces must be considered. In some cases, the process is favored by one driving force and not favored by the other. In such cases, a temperature determines whether the reaction is spontaneous.
In 1873, American scientist Josiah Willard Gibbs proposed a function that came to be known as Gibbs free energy. Gibbs free energy, represented by the symbol G, is a property of a system that depends on the enthalpy (H), entropy (S), and Kelvin temperature (T) of the system:
G = H - T•S
Technically speaking, Gibbs free energy is the amount of energy that can be freed or extracted from the system in order to do useful work. But for us, Gibbs free energy becomes a useful mathematical tool for predicting the spontaneity of a reaction. Let’s learn how it works.
Gibbs Free Energy Change
Under standard conditions, the Gibbs free energy value is different for reactants and products for a reaction. The quantity is a state function. The significance of this is that a reaction has a change in Gibbs free energy that is only dependent upon the identity of the reactants and the products (and their properties, such as temperature, pressure, and concentrations). The Gibbs free energy change, ∆G, does not depend upon the path by which the reactants turn into products. It can be calculated using the equation:
∆G = ∆H - T•∆S
If the enthalpy change (∆H) of the system, the entropy change (∆S) of the system, and the Kelvin temperature (T) are known, the Gibbs free energy change can be calculated.
We see both driving forces for spontaneous change in this equation. As we learned in Lesson 2d, the ∆H term is the driving force that primarily affects the change in entropy of the surroundings. A negative ∆H value favors the process being spontaneous. The T•∆S term is the driving force that expresses the entropy of the system. A positive value for T•∆S favors the process being spontaneous. When both driving forces for spontaneous change are favorable, the value of ∆G is negative. In general, we can make the claim that all spontaneous processes will have a negative ∆G value. And conversely, processes with a positive ∆G value are not spontaneous. Non-spontaneous processes include those with a positive ∆H and a negative ∆S value.
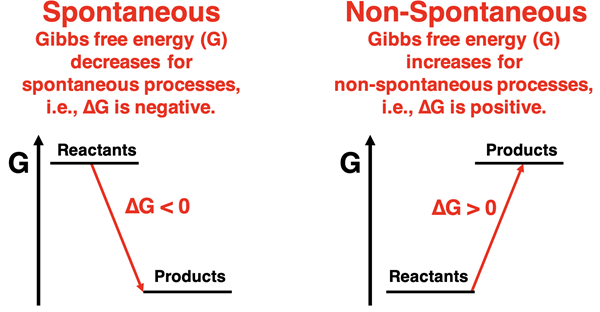
Predicting Spontaneity When Driving Forces are in Opposition
The Gibbs free energy equation identifies two driving forces. The enthalpy change (∆H) is one driving force; we described this driving force as energy spread or ∆Ssurroundings in Lesson 2. The entropy change of the system is the other driving force; we described this driving force as matter spread and ∆Ssystem in Lesson 2. These two driving forces are sometimes aligned such that the process is clearly spontaneous or clearly non-spontaneous. But on other occassions, the two driving forces are in opposition, with one favoring the spontaneous process and the other opposing it. What then? How do you reason through the spontaneity question when there is no agreement between both driving forces?
Temperature becomes the critical variable when the two driving forces are in opposition. The Kelvin temperature is the variable T in the Gibbs free energy change equation. Any T value that results in a negative ∆G will be a temperature for which the process is spontaneous. Think it through like this, knowing that the entropy change will be multiplied by a temperature value:
- If ∆H is - and ∆S is -, then the entropy change (∆S) does not favor the process being spontaneous. A lower temperature will make the T∆S term smaller and minimize the effect of entropy change. The reaction is spontaneous at lower temperatures - from 0 K to some threshold value (which we will later learn to calculate).
- If ∆H is + and ∆S is +, then the enthalpy change (∆H) does not favor the process being spontaneous while the entropy change (∆S) does. A higher temperature will make the T∆S term larger and maximize the effect of entropy change upon spontaneity. The reaction is spontaneous at higher temperatures - at some threshold value (which we will later learn to calculate) and above.
The following table summarizes the relationship between the signs on ∆H, ∆S, ∆G, and spontaneity.
Gibbs Free Energy Change as Reaction Potential

We can think of a quantity like Gibb’s free energy change as having a magnitude and a sign (+ or -). By magnitude, we mean
size or
greatness. A ∆G value of -100 kJ has a greater magnitude than a ∆G value of -10 kJ. When making this judgement, we are extracting the negative from the ∆G value and saying 100 kJ is greater than 10 kJ. In effect, we are saying that the negative tells us that the process is spontaneous and the 100 or the 10 tells us something else. What does it tells us?
Think of a ∆G value as an indicator of
reaction potential. A larger magnitude and a negative sign indicates that the reaction proceeds strongly in the forward direction. A small magnitude and a negative sign indicates that the reaction proceeds in the forward direction, but with a bit more
pushback.
You can relate this concept of magnitude and sign to
reversible systems and equilibrium. (And that’s what
our next page is about.) A large magnitude, negative ∆G value can be compared to a reaction that proceeds nearly to completion. A small magnitude, negative ∆G value can be compared to a reaction that proceeds in the forward direction and reaches an equilibrium before all reactants are used up. Finally, a positive ∆G value indicates that the process is not spontaneous. However, it also indicates that the reverse of the process is spontaneous. That is, the products (if present) would spontaneously turn into reactants.
We can summarize this as follows:
- If ∆G is negative, the reaction is spontaneous; the greater the magnitude of the value, the more strongly it occurs
- If ∆G is positive, the reaction is non-spontaneous. However, the reverse of the reaction is spontaneous.
- If ∆G is 0, then the reaction is at an equilibrium.
Calculating ∆G to Predict Spontaneity
The Gibbs free energy change equation can be used to calculate the ∆G value for a chemical or physical change. The calculated value allows one to predict if the reaction is spontaneous. To perform the calculation, a ∆H value, a ∆S value, and a Kelvin temperature is required. If a celsius temperature is known, the Kelvin value can be determined by adding 273.15 to the celsius value.
T(K) = T(°C) + 273.15
Like any calculation in Chemistry, attention to the units is important. It is especially critical since values of ∆H are usually given in kilojoule (kJ) and values of ∆S are usually given in joule per Kelvin (J/K). The energy units must match. Knowing that 1000 J = 1 kJ allows you to change a quantity in J to kJ by dividing by 1000.
Example 1
Consider the formation of nitrogen dioxide from its elements as shown.
N2(g) + 2 O2(g) → 2 NO2(g)
Given that ΔH = 68 kJ and that ΔS = -122 J/K, ...
a. Calculate the free energy change of this reaction at 566 K.
b. Is the reaction spontaneous at this temperature?
Solution
Part a:
All quantities - ∆H, ∆S, and T - are given. We will use -0.122 kJ/K for the ∆S value so its energy unit matches that of ∆H. Substitution and algebra proceeds as follows:
∆G = 68 kJ - (-0.122 kJ/K)•(566 K)
∆G = 68 kJ - (-69.052 kJ)
∆G = 137 kJ
Part b:
The reaction is not spontaneous since the ∆G value is positive.
Example 2
Calculate the ∆G values for the decomposition of calcium carbonate (calcite) at the given temperatures. Complete the provided table.
CaCO3(s) → CaO(s) + CO2(g)
Given: ∆H = +178.5 kJ and ∆S = +158.1 J/K
Solution
For all three rows, we will use +0.1581 kJ/K as the ∆S value so that its energy unit matches that of ∆H. The Kelvin temperature will be used in the calculation. We will add 273 to the Celsius temperatures.
Part a:
∆G = +178.5 kJ - (+0.1581 kJ/K)•(293 K)
∆G = +178.5 kJ - (+46.3 kJ)
∆G = +132.2 kJ
Not Spontaneous (due to + sign on ∆G)
Part b:
∆G = +178.5 kJ - (+0.1581 kJ/K)•(1373 K)
∆G = +178.5 kJ - (+217.1 kJ)
∆G = -38.6 kJ
Spontaneous (due to - sign on ∆G)
Part c:
∆G = +178.5 kJ - (+0.1581 kJ/K)•(1473 K)
∆G = +178.5 kJ - (+232.9 kJ)
∆G = -54.4 kJ
Spontaneous (due to - sign on ∆G)
Here is a summary of the results:
Determining a Threshold Temperature
In situations in which there are competing driving forces, the temperature makes the difference between spontaneity and non-spontaneity. To determine the threshold temperature, above or below which the reaction is spontaneous, the ∆G value can be set to 0 and the value of
Tthreshold can be determined. The equation becomes:
Tthreshold = ∆H/∆S
Once a threshold temperature is determined, good reasoning must be used to determine if the process is spontaneous above the threshold temperature or below the threshold temperature. We have modeled that reasoning above in the section titled
Predicting Spontaneity When Driving Forces are in Opposition.
Example 3
For the following reaction, the values of ΔH and ΔS are -58.03 kJ and -176.6 J/K, respectively.
2 NO2(g) → N2O4(g)
a. What is the value of ΔG° at 274 K? Is the reaction spontaneous at this temperature?
b. At what temperature (in °C) is ΔG° = 0?
c. Is ΔG° negative above or below this temperature?
Solution
Part a:
∆G = -58.03 kJ - (-0.1766 kJ/K)•(274 K)
∆G = -58.03 kJ - (-48.39 J/K)
∆G = -9.64 kJ
Spontaneous (due to - sign)
Part b:
Use Tthreshold = ∆H/∆S
Tthreshold = (-58.03 kJ) / (-0.1766 kJ/K)
Tthreshold = 328.6 K
Tthreshold = 55.4°C (subtract 273.15 from the Kelvin value)
Part c:
Spontaneous (∆G is negative) below this threshold temperature
The ∆S value is negative, a non-favorable condition for spontaneity. So smaller temperatures make the T•∆S term smaller and minimize the effect of the non-favorable ∆S driving force. (Besides, we saw in Part a that the reaction was spontaneous at 274 K ... a temperature below the threshold.)
Example 4
For 1 mole of mercury, the enthalpy of vaporization is +58.51 kJ and the entropy of vaporization is +92.92 J/K. Based on this data, what is the normal boiling point (in °C) of mercury?
Solution
The boiling point temperature is the threshold temperature. The system is at equilibrium at this temperature and ∆G is 0. The calculation of the boiling point uses the equation T
threshold = ∆H/∆S. Here’s the work.
Tthreshold = ∆H/∆S = (58.51 kJ) / (0.09292 kJ/K)
Tthreshold = 629.7 K
Tthreshold = 356.5°C (subtract 273.15 from the Kelvin value)
Next Up
We will finish our chapter on Thermodynamics by discussing the connection between our Gibbs free energy model and the equilibrium model presented in
Chapter 14 of our
Chemistry Tutorial. But before you leave, try one or more of the practice and reinforcement suggestions mentioned below.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Try our Concept Builder titled Gibb's Free Energy. Any one of the three activities provides a great follow-up to this lesson.
Check Your Understanding of Gibbs Free Energy Change
Use the following questions to assess your understanding of Gibbs free energy and the use of the equation to predict spontaneity. Tap the Check Answer buttons when ready.
1. TRUE or FALSE:
If a reaction is known to have a negative ∆G value, then one can be certain that it is spontaneous regardless of the sign and value of ∆H, ∆S, and T.
2. Given the following ∆H and ∆S values for a chemical reaction, identify the reaction as being either ...
- Spontaneous at all temperatures
- Not spontaneous at any temperature
- Spontaneous only in a low temperature range
- Spontaneous only in a high temperature range
a. ∆H = +345 kJ and ∆S = -213 J/K
b. ∆H = -426 kJ and ∆S = -128 J/K
c. ∆H = -521 kJ and ∆S = +109 J/K
d. ∆H = +249 kJ and ∆S = +76 J/K
3. Consider the following phase change:
H2O(l) → H2O(g)
having ∆H and ∆S values of …
∆H = +40.7 kJ
∆S = +109.1 J/K
Calculate the ∆G value (to the first decimal place) at temperatures of
a. 98°C
b. 102°C
c. 200°C
4. The enthalpy change (ΔH), entropy change (ΔS), and Kelvin temperature for a particular process is …
ΔH = +158.2 kJ
ΔS = +584.4 J/K
T = 55°C
a. Calculate the free energy change (ΔG) of this process.
b. Is this reaction spontaneous at this temperature?
5. A substance has an enthalpy of vaporization of +206.5 kJ/mol and an entropy of vaporization of +568.4 J/K/mol. Calculate the normal boiling point (in °C) of the substance.
6. Consider the reaction for the synthesis of sulfur dioxide from its elements.
S(s) + O2(g) → SO2(g)
Given: ∆H = -296.8 kJ and ∆S = +10.9 J/K
Can you determine the threshold temperature for which the ∆G is greater than zero (and the reaction is not spontaneous)? Conduct some calculations and discuss your findings.