Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Spontaneity and Entropy
Part a: Driving Forces for Change
Part a: Driving Forces for Change
Part b:
What is Entropy?
Part c:
Mathematics of Entropy Change
Part d:
The Second Law of Thermodynamics
The Big Idea
Every spontaneous process is guided by forces that push energy and matter toward more dispersed states. These driving forces set the stage for understanding entropy and why change happens.
What Forces Drive a Reaction to Occur?
In Lesson 1b, we learned that a spontaneous process is a process that occurs naturally on its own without any sustained or continued intervention. The rusting of iron, while slow, is a spontaneous process that requires no intervention. This is in contrast to the decomposition of water into its elements, a nonspontaneous process that requires a continuous input of energy. The question now arises What makes a process spontaneous? Why does one reaction occurs on its own while another reaction requires sustained and continuous intervention?
In Lesson 2, we will address the question of what factors promote the spontaneity of a reaction. That is, what are the driving forces for a reaction? This question will lead us to a new concept known as entropy in Lesson 2b, and eventually to the second law of thermodynamics in Lesson 2d. On this page, we will look rather broadly at two concepts - energy spread and matter spread - as being driving forces for spontaneous change.
From Chicago to Cleveland on a Tank of Gas
Let’s begin with a thought experiment. Suppose you plan a road trip from Chicago to Cleveland to visit a friend. You begin the trip by filling the tank of your car with 14 gallons of octane gasoline. You arrive in Cleveland six hours later with a near-empty gas tank. We understand and believe in the first law of thermodynamics - that the total energy in the universe is constant. So, what happened to all the energy that was originally concentrated in the octane gasoline in the gas tank?
A car is equipped with an internal combustion engine (as of this writing) in which octane (C8H18) undergoes an exothermic, combustion reaction:
2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(g) + 11500 kJ
The engine transforms the stored chemical energy to thermal energy. The hot product gases (CO2 and H2O) expand. A sophisticated mechanism involving pistons, crankshafts, axles, and wheels transforms the thermal energy into the kinetic energy of the car while the hot exhaust gases exit through the tailpipe. With the car set in motion, you make it to Cleveland. When you arrive, you park in your friend’s driveway. There’s little to no energy remaining in the gas tank and you and your hot car have zero kinetic energy.
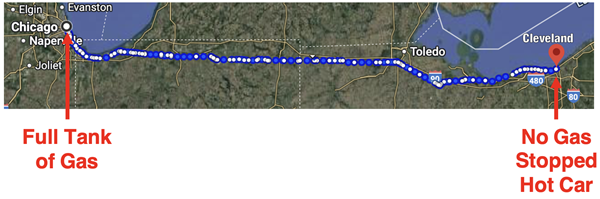
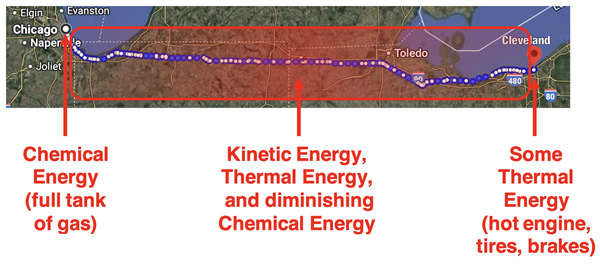
 If the total energy originally present in the gasoline is conserved, then where is it at now? What happened to the chemical energy that was concentrated in the 14 gallons of octane? The short answer is: it’s all over the place. While the engine transformed the chemical energy into kinetic energy to get you and your car to Cleveland, all of that original energy is eventually dissipated as heat. That heat is spread all over the place between Chicago and Cleveland ... and it is still spreading all over the universe.
If the total energy originally present in the gasoline is conserved, then where is it at now? What happened to the chemical energy that was concentrated in the 14 gallons of octane? The short answer is: it’s all over the place. While the engine transformed the chemical energy into kinetic energy to get you and your car to Cleveland, all of that original energy is eventually dissipated as heat. That heat is spread all over the place between Chicago and Cleveland ... and it is still spreading all over the universe.
Some of the heat escaped with the hot exhaust gases out the tailpipe between Chicago and Cleveland. You did some stopping along the way and that means your brakes warmed up. They don’t stay hot for long, but instead interact with surrounding air to warm the air up ... between Chicago and Cleveland. And all along the way, the rubber tires interact with the road due to friction and both the tires and the road warm up. Like hot brakes, the road and tires interact with the surrounding air and warm the air up ... between Chicago and Cleveland. When you finally arrive in Cleveland, all that is left in the car of the original 14 gallons of octane energy is a hot engine; and that thermal energy will soon be dissipated to the surrounding air.
Energy Spread
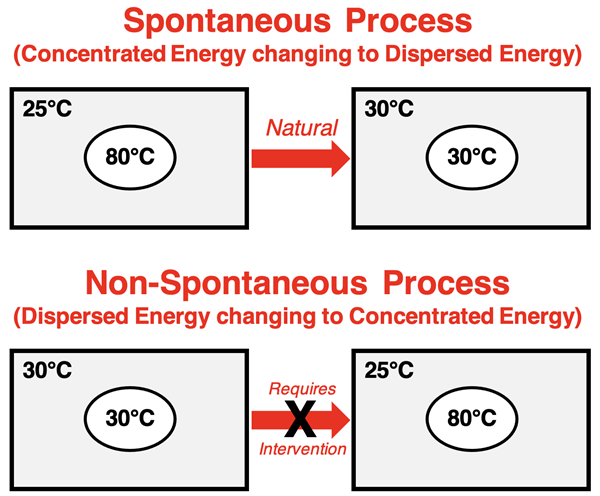
So what does this thought experiment, repeated in real life countless times every day, tell us? It tells us that nature always tends towards a change from concentrated energy (14 gallons of octane gasoline) to dissipated or dispersed energy (thermal energy spread between Chicago and Cleveland). Energy naturally and spontaneously spreads. It is natural for a hot object to cool down by transferring its energy to the surrounding objects. The once-hot object and the surrounding air eventually reach a thermal equilibrium in which they have the same temperature.
You can be certain that the opposite direction of change will never happen: a same-temperature object present in air will not spontaneously warm up while the air cools down. Nature always heads in one direction: from concentrated energy to dispersed energy. Energy spread is the rule ... or at least, one of the two rules. Energy spread is one of the two driving forces for a spontaneous processes.
Endothermic vs. Exothermic Processes
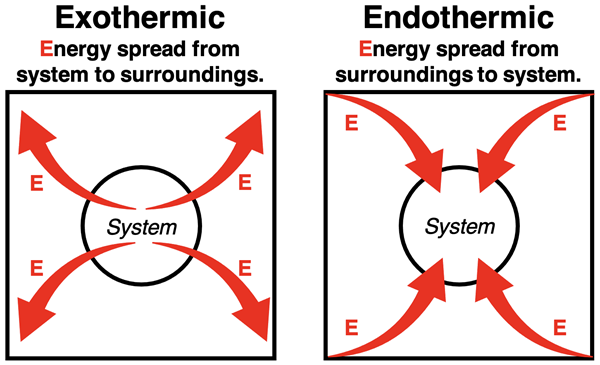
We can relate this idea of energy spread to endothermic and exothermic processes. Exothermic processes involve the dispersal or spread of energy. Energy is released from the system to the surroundings (the rest of the universe). When an exothermic reaction occurs, chemical energy concentrated within the system of chemicals is released as heat to the rest of the universe.
Endothermic processes involve the concentrating of energy. Heat from the surroundings cross the system boundary into the system. When an endothermic reaction occurs, energy from the surroundings (the universe) enters the system and becomes concentrated as internal energy within the system.
Nature favors exothermic processes. Nature does not favor endothermic processes. Does this mean an endothermic process will never occur spontaneously? And that exothermic processes always occur spontaneously? No! And No! There are two driving forces. And both driving forces must be considered. If the first driving force is referred to as energy spread, then the second driving force can be referred to as matter spread.
Matter Spread
Consider a metal container filled with a gas placed in the middle of a larger room. The gas is concentrated in a relatively small amount of space. If the lid of the container is opened, the gas will naturally spread on its own to fill the entire room. This is a spontaneous process. Matter (stuff, atoms, etc.) has spread or dispersed to larger expanse of space.
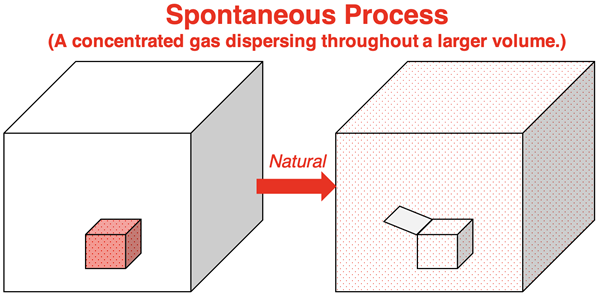
Would the opposite ever occur? Would gas dispersed about a room move about and gather together such that it is all concentrated inside the small container? The opposite process of concentrating matter is not spontaneous. Matter spread is the rule ... or at least the second of two rules. Matter spread is the second driving force for a spontaneous process.
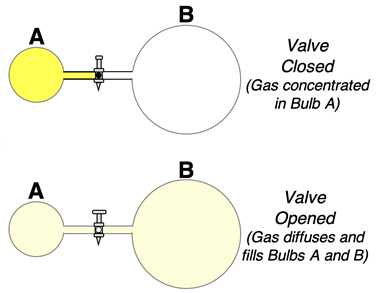
As a second example, consider a two-bulb system separated by a valve like that shown, with all the gas initially present in the Bulb A. If the valve is opened, some gas from Bulb A will diffuse to Bulb B. After some time is allowed, all the gas will be dispersed uniformly throughout the two-bulb system. This spread of matter is spontanteous and occurs in one direction. The reverse - matter concentration - is never expected.
Our two examples of matter spread involve the dispersal of a gas. Gas dispersion is a consequence of the nature of a gas to be in constant, random motion, colliding with each other and with the walls of the container. This random particle motion in the absence of interparticle forces is what causes a gas to fill the container that it is in.

Matter spread is not restricted to gaseous states of matter. Dissolving of solids in water results in the dispersal of matter about the solution. For instance, when blue copper sulfate
crystals - CuSO4 - are dissolved in water, the ionic compound dissociates and the blue copper ions are dispersed about the entire container. This too is matter spread and will occur spontanteously, even in the absence of stirring.
Matter spread is often described as a process in which the elements of a system - whether a chemical system or some physical system - become more disordered and disorganized. Take a deck of 52  playing cards, with the cards organized by suit; that is, all the hearts are together, all the clubs are together, etc. If tossed across a table and gathered together again, the cards will likely no longer be ordered by suit. And if a deck of disordered cards is tossed across a table and gathered together again, the likelihood that they will be ordered by suit is statistically improbable. This concept of disorder and probability will be discussed in detail on our next page when we introduce the concept of entropy.
playing cards, with the cards organized by suit; that is, all the hearts are together, all the clubs are together, etc. If tossed across a table and gathered together again, the cards will likely no longer be ordered by suit. And if a deck of disordered cards is tossed across a table and gathered together again, the likelihood that they will be ordered by suit is statistically improbable. This concept of disorder and probability will be discussed in detail on our next page when we introduce the concept of entropy.
Source: Playing Cards (Public Domain)
Melting and Freezing
Energy spread and matter spread are the two driving forces for spontaneous processes. Nature favors processes that spread energy as widely as possible and matter as widely as possible. Processes that spread both energy and matter will be spontaneous processes. But what happens when there is a process in which energy spreads but matter concentrates? Or what happens when a process concentrates energy but spreads matter? As we will learn later in this lesson, when the two driving forces compete against each other, temperature becomes the tie-break variable. This is best demonstrate if we consider the processes of the melting of ice and the freezing of liquid water.
The melting of ice is an endothermic process. Energy spread does not favor the melting of ice. But when ice melts, the intermolecular forces that hold water molecules in an organized crystal are weakened and water molecules are freed up to move. That means that melting ice results in matter spread. The two driving forces are in competition with one another.
Contrast ice melting with the opposite process - the freezing of liquid water. The freezing of water is an exothermic process. Energy spread favors water freezing. But when liquid water freezes, its molecules organize into a crystal with strong intermolecular forces that prevent translational motion. There is no matter spread. The two driving forces are in competition with one another.

So, what does water do - freeze or melt? The answer is associated with temperature. Temperature becomes the tie-break variable. At temperatures below 0°C, liquid water will freeze. But at temperatures above 0°C, the freezing of liquid water is not spontaneous. On the other hand, ice will melt at temperatures above 0°C but it will not melt below 0°C.

We will discuss situations like these in more detail as we progress through Lesson 2. And in Lesson 3, we will learn how to calculate the tiebreak temperature.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Driving Forces for Change. Save it to a safe location and use it as a review tool.
Check Your Understanding of Driving Forces
Use the following questions to assess your understanding of the factors that promote spontaneity. Tap the Check Answer buttons when ready.
1. The word dissipate or dissipated was used quite frequently on this page and will be used frequently on subsequent pages of this lesson. Review the meaning of the word and use it in a sentence.
2. Del Ekatessen bakes some banana bread in the oven. She uses the energy stored in methane gas (CH
4) to bake the bread. Describe where the energy is at 24 hours later. Identify as many places or forms as you can.
3. Which of the following processes are examples of energy spread? Select all that apply.
a. The boiling of water.
b. The combustion of a candle.
c. The cooling of a hot teapot on the stove.
d. The burning of leaves in a bonfire.
4. Which of the following processes are examples of matter spread? Select all that apply.
a. A cup of water spills on the countertop.
b. Salt is dissolved in water to make a saline rinse.
c. A snow plow clears the street and moves the snow to the curb.
d. You sneeze and fail to cover your mouth in time.
5.
TRUE or
FALSE:
- Exothermic reactions are always spontaneous.
- Chemical reactions that involve the spread of matter are always spontaneous.
- A reaction that releases heat to the surroundings is one that has a favorable energy spread.
- A reaction that involves both energy spread and matter spread will be spontaneous.
- When water boils, there is a favorable energy spread.
- When water boils, there is a favorable matter spread.