Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Equilibrium
Part a: The Equilibrium State
Part a: The Equilibrium State
Part b:
Equilibrium Constant Expressions
Part c:
Calculations of K and Concentration
Part d:
Predicting the Direction of Reaction
Part e:
Analyzing Equilibrium Systems
The Big Idea
Chemical equilibrium refers to a dynamic state in which the forward and reverse reaction rates are equal, so concentrations of reactants and products remain constant — though there is still change occurring at the molecular level.
Reversible Systems
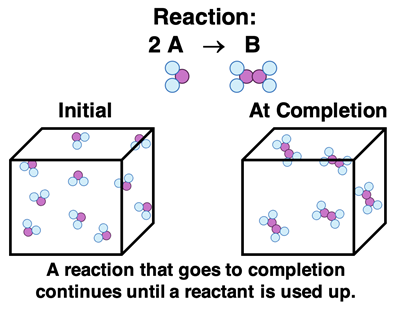
So far in this Chemistry Tutorial we have considered only reactions that go to completion. That is, we’ve considered every reaction to continue until the limiting reactant is used up. Once one or more of the reactants is consumed, the reaction stops. We say that the reaction has gone to completion. It is over. Complete. Done. When a reactant is used up, there can be no reaction and no more products can be produced.
This chapter will focus on a different type of reaction system - a reversible reaction system. An example would include the reaction described by the following balanced equation.
2 NO2(g) ⇄ N2O4(g)
brown colorless
The reactant is a reddish-brown gas. The product is a colorless gas. Suppose you fill a glass tube with the reddish-brown gas (NO2) and seal it. Over time, the NO2 will react and form the colorless product - N2O4. A it does, you will observe an indisputable change in the intensity of the brown color. As the NO2 reacts to form the colorless N2O4, the gas in the tube begins to fade in color since there is less of the brown gas and more of the colorless gas. But at room temperature, you will never observe the pure colorless gas. Why? Because the reaction does not go to completion. Instead, the reaction reaches a so-called equilibrium state in which there is no more observable change in the amount of reactants and products.
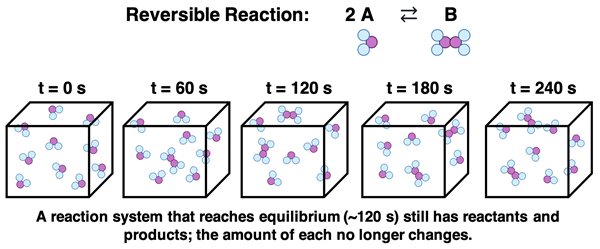
Reactions that do not go to completion but instead reach an equilibrium are reversible reaction systems. A reversible reaction system is indicated by a different reaction symbol (⇄) in the balanced chemical equation. The ⇄ indicates that the reaction occurs in both directions … simultaneously. Reactants can form products and existing products can from reactants. Think of it as a two-way reaction or a bi-directional reaction. We would refer to A forming B as the forward reaction (→) and B forming A as the reverse reaction(←).
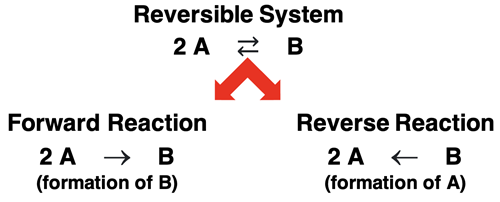
 If the brown NO2 gas in the glass tube reacted to completion, the tube would eventually be colorless and the N2O4 product is all that would be present once completion was reached. But the NO2 - N2O4 system is reversible. The NO2 turns to N2O4 and the N2O4 turns back into NO2. The reverse reaction is the game changer. It opposes the forward reaction. With two opposing processes occurring at the same time, completion is never reached. Instead, the system reaches an equilibrium position. Equilibrium means balance or steadiness. The reversible system achieves equilibrium because two opposing processes are balancing each other. Once the balance is achieved (that is, once equilibrium is reached), there is no more observable change in the amount of reactants and products. The color of the tube remains fixed, but it is neither the colorless N2O4 nor the reddish-brown NO2.
If the brown NO2 gas in the glass tube reacted to completion, the tube would eventually be colorless and the N2O4 product is all that would be present once completion was reached. But the NO2 - N2O4 system is reversible. The NO2 turns to N2O4 and the N2O4 turns back into NO2. The reverse reaction is the game changer. It opposes the forward reaction. With two opposing processes occurring at the same time, completion is never reached. Instead, the system reaches an equilibrium position. Equilibrium means balance or steadiness. The reversible system achieves equilibrium because two opposing processes are balancing each other. Once the balance is achieved (that is, once equilibrium is reached), there is no more observable change in the amount of reactants and products. The color of the tube remains fixed, but it is neither the colorless N2O4 nor the reddish-brown NO2.
Concentration vs. Time for Reversible Systems
Suppose we had a means of measuring the molar concentrations of NO2 and N2O4 in our reversible reaction system. How would we observe the concentrations of the two species (i.e., the reactant and product) to change over time? The plot below tells the story.
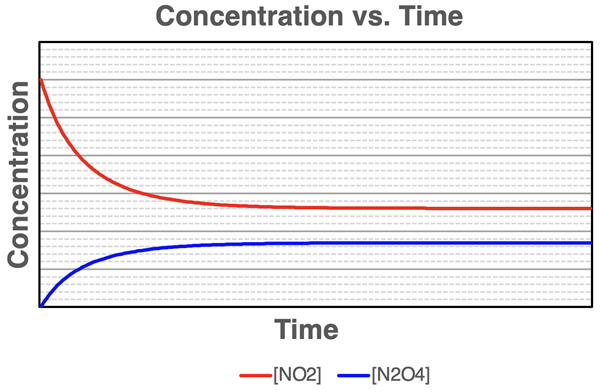
 Initially, there is only NO2 in the glass tube. This NO2 begins to react away and N2O4 is formed. The concentration of NO2 (red line on graph) begins to decrease relatively rapidly at first. As time progresses, the slope of the line decreases and eventually the concentration of NO2 becomes constant or steady. A similar trend is observed for the N2O4 line (blue line). The concentration of N2O4 is initially zero. The N2O4 line slopes upward relatively steeply at first and gradually levels off to a constant or steady concentration level. At equilibrium, the concentrations of reactants and products are constant. The concentrations are not equal to each other, but they are constant.
Initially, there is only NO2 in the glass tube. This NO2 begins to react away and N2O4 is formed. The concentration of NO2 (red line on graph) begins to decrease relatively rapidly at first. As time progresses, the slope of the line decreases and eventually the concentration of NO2 becomes constant or steady. A similar trend is observed for the N2O4 line (blue line). The concentration of N2O4 is initially zero. The N2O4 line slopes upward relatively steeply at first and gradually levels off to a constant or steady concentration level. At equilibrium, the concentrations of reactants and products are constant. The concentrations are not equal to each other, but they are constant.
There is a subtle difference in the amount of concentration change for NO2 and N2O4. The change in [NO2] is twice the change in [N2O4]. This is consistent with the stoichiometry of the reaction. 1 mole of N2O4 is produced for every 2 moles of NO2 that react.
2 NO2(g) ⇄ N2O4(g)
Rate vs. Time for Reversible Systems
The graph below displays the rates of the forward and reverse reactions as a function of time for the NO2 - N2O4 system. For consistency, these rates are defined as the rate at which NO2 is reacted by the forward reaction and the rate at which NO2 is produced by the reverse reaction.
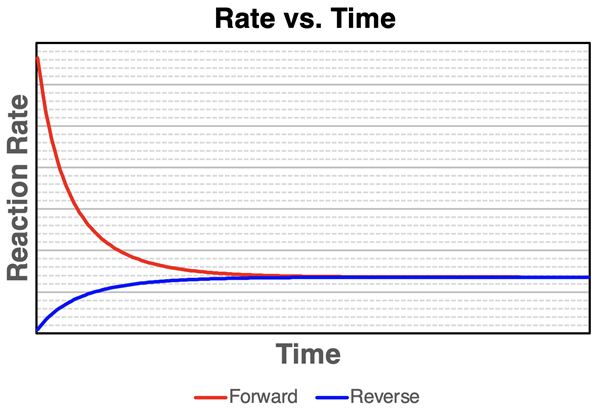
Reaction rates depend upon concentrations of the reactants. As concentrations increase, the reaction rates increase. And as concentrations decrease, the reaction rates decrease. This explains the shapes of the two curves on the graph. The forward reaction rate (red line) depends upon [NO2]. The system initially contains only NO2. Since [NO2] is high, the rate of the forward reaction is very high. But as NO2 reacts away, the [NO2] decreases, and the rate of the forward reaction likewise decreases.
 The reverse reaction (blue line) depends upon the [N2O4]. Initially, there is no N2O4 in the system so the rate begins at 0. As N2O4 is produced by the forward reaction, the [N2O4] begins to increase and the rate of the reverse reaction likewise increases. The rate of the reverse reaction continues to increase as more N2O4 is produced by the forward. Eventually the rate of the forward reaction is equal to the rate of the reverse reaction. It is at this point that the N2O4 is being reacted away at the same rate that it is being produced. The same can be said for the NO2. Equilibrium is reached once the forward and reverse reactions are occurring at the same rate.
The reverse reaction (blue line) depends upon the [N2O4]. Initially, there is no N2O4 in the system so the rate begins at 0. As N2O4 is produced by the forward reaction, the [N2O4] begins to increase and the rate of the reverse reaction likewise increases. The rate of the reverse reaction continues to increase as more N2O4 is produced by the forward. Eventually the rate of the forward reaction is equal to the rate of the reverse reaction. It is at this point that the N2O4 is being reacted away at the same rate that it is being produced. The same can be said for the NO2. Equilibrium is reached once the forward and reverse reactions are occurring at the same rate.
These two graphs highlight the two conditions of chemical equilibrium.
- The concentrations of reactants and products are held steady or constant.
- The rate of the forward reaction is equal to the rate of the reverse reaction.
These two statements are always true of any system that has reached equilibrium.
What Does it Mean to Describe Equilibrium as a Dynamic State?
It is important to recognize that equilibrium is a dynamic state. That is, the reactions are still occurring. The N
2O
4 product is still being produced once equilibrium is established. It is just reacting away at the same rate as it is being produced. A reaction is considered a chemical change. And at equilibrium there is a whole lot of changing going on. It’s just that one change (the production of N
2O
4) is offset by the other change (the reacting away of N
2O
4) such that the [N
2O
4] remains constant. This is what is meant in saying that equilibrium is a dynamic state.
An analogy may help to solidify the concept of a dynamic equilibrium. Consider a high school that consists of two side-by-side buildings with a single passageway connecting the buildings. Classes are held in both buildings. On a day full enrollment, there are 1000 students in Building A and 500 students in Building B. During the passing period between classes, students use the passageway to move between buildings. Suppose there are 200 students moving from Building A to their next class in Building B. And there are 200 other students in Building B moving to their next class in Building A. The rates at which students move between buildings in opposite directions is equal. The result is that the number of students in Building A does not change. And the number of students in Building B does not change.
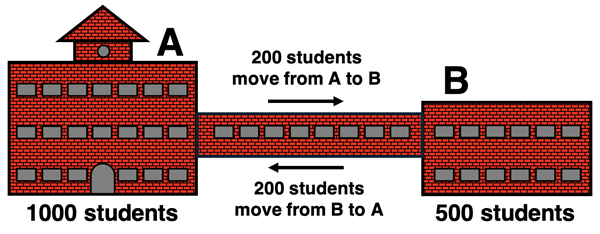
Chemical equilibrium is very much like the school building analogy. There is change going on. It’s dynamic. There’s two opposing processes - a forward reaction and a reverse reaction. But because these opposing processes occur at the same rate, there is no noticeable change in the amount of reactants and products. When a system has reached equilibrium, the two opposing processes have
balanced each other.

When a reversible reaction system has reached equilibrium, it is improper to say that the reaction has stopped. The reaction is still occurring! The only thing that has stopped is the change in concentrations of reactants and products. Concentrations remain steady when a system reaches equilibrium.
What is an Equilibrium Position?
In our discussion of the NO
2 - N
2O
4 reaction system, we started with all NO
2 and no N
2O
4. What would happen if the system contained only N
2O
4? The graphs below tell the story.
The system would still reach equilibrium. The process by which it does is different but the end result is that equilibrium is reached. In this case, the rate of the reverse reaction is initially greater than the rate of the forward reaction. This causes the N
2O
4 to decrease and the [NO
2] to increase. As [N
2O
4] decreases, the reverse reaction rate slows down over the course of time. And as [NO
2] increases, the forward reaction rate speeds up. Eventually the rate of the forward and reverse reaction balance each other and the concentrations remain steady.
Now suppose we start with equal amounts of NO
2 and N
2O
4. What would happen? Once more, the graphs tell the story.
The reversible system once again establishes an equilibrium. This is always the case. There is no set of conditions that could be set up to prevent a reversible system from eventually establishing an equilibrium.
Now suppose we run several controlled trials with our NO
2 - N
2O
4 reaction system in which we vary the initial concentrations and measure the equilibrium concentrations of NO
2 and N
2O
4. Each trial will be run at the same temperature. The table below shows the results.
In each trial, the system attained an equilibrium. It did not matter how we initially set up the system. We could overload the system with NO
2, overload with N
2O
4, provide equal amounts of both NO
2 or N
2O
4, or anything in between … and still the system responds so as to reach equilibrium.
The set of two equilibrium concentrations - [NO
2] and [N
2O
4] - varied from trial to trial. Each particular set is referred to as an
equilibrium position. This term refers to the set of final concentrations for every reactant and product in the system. For every reversible system, there is an infinite number of sets of concentrations for which equilibrium is established. The exact values depend only on the initial concentrations, the temperature, and the actual reaction. At this point in Lesson 2, there is likely no obvious mathematical relationship between these concentrations. But as we will see in
Lesson 2b, each set of concentrations share the same mathematical relationship.
Learning the Language of Equilibrium
There are a group of terms that will be used through the remainder of Lesson 2 and again in
Lesson 3 that a student of Chemistry needs to become comfortable with. The terms describe how the reaction system responds in order to achieve its equilibrium. The terms are:
- Proceeds to the right or (proceeds in the direction of the forward reaction)
- Proceeds to the left or (proceeds in the direction of the reverse reaction)
A reaction that proceeds to the right will display a decrease in reactant concentration and an increase in the product concentrations. A reaction that proceeds to the left will display an increase in reactant concentration and a decrease in the product concentrations. Each trial in the table above is analyzed below to illustrate the concept of which direction a reaction proceeds to reach equilibrium. Analyze the graphic and begin to accustom yourself with the use of this language.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Try our Concept Builder titled The Equilibrium Concept. Any one of the three activities provides a great follow-up to this lesson.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on the Equilibrium Concept. Save it to a safe location and use it as a review tool.
Check Your Understanding of the Equilibrium State
Use the following questions to assess your understanding of concepts and terminology associated with chemical equilibrium. Tap the Check Answer buttons when ready.
1. If a reaction does not proceed towards completion, then it is likely a _______ reaction system.
- irreversible
- reversible
- limiting
- unbalanced
2. When reactions are said to be
reversible it means that _____.
- the damage done by the reaction is not final; one can find a way to reverse the damages
- the reaction is like a convertible; it changes or converts one set of chemicals to another set
- reactants turn to products; but once complete, those products can be turned back into reactants
- reactants turn into products but the reverse can occur at the same time: products can turn to reactants
3. Describe the two conditions that exist when a reaction system has reached equilibrium.
4. Which of the following are TRUE of a reaction system that has reached equilibrium? Select all that apply.
a. Products are no longer being formed.
b. Concentrations of reactants are equal to the concentrations of products.
c. The rate of the forward reaction is equal to the rate of the reverse reaction.
d. Concentrations of reactants and products have reached their maximum level.
e. Concentrations of reactants and products have become steady and constant.
f. The rate of both the forward and the reverse reaction is 0.
5. What is the significance of saying an equilibrium state is a dynamic state?
a. It is a very powerful and exuberant state. Everybody enjoys observing it.
b. There is a lot of change still occurring. Reactants are turning into products and vice versa.
c. It took a lot of effort to be reached. Both the forward and the reverse reaction occurred for some time before it was attained.
d. The concentrations of reactants and products are still undergoing dynamic change. There is no telling what they will be in a minute from now.
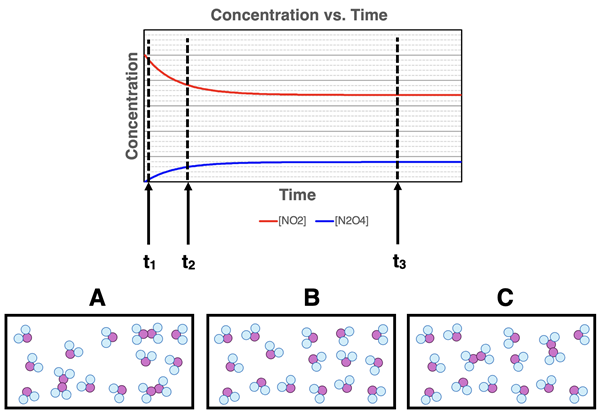
6. An NO
2-N
2O
4 system starts with all NO
2. Eventually it reaches equilibrium. A plot of concentration vs. time is shown Three particle diagrams are also shown. Math the particle diagrams A, B and C to times t
1, t
2, and t
3 on the graph.
7. Complete this paragraph:
Closed, reversible systems undergo change until they reach equilibrium. If the system
initially consists of only reactants, then the forward reaction procedes at a ______________ (higher, lower) rate than the reverse reaction. Concentrations of reactants begin to ______________ (increase, decrease) while concentrations of products ______________ (increase, decrease). This causes the forward reaction to ______________ (speed up, slow down) and the reverse reaction to ______________ (speed up, slow down). These changes continue until the rates of the forward and reverse reaction __________________________ ( are equal to each other, are equal to 0, are no longer changing). When this occurs, equilibrium is established and the concentrations of reactants and products __________________________ ( are equal to each other, are equal to 0, are no longer changing).