Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Spontaneity and Energy
Part b: Spontaneous Processes
Part a:
What is Thermodynamics?
Part b: Spontaneous Processes
Part c:
The First Law of Thermodynamics
The Big Idea
Some changes occur naturally on their own — without sustained intervention. Spontaneous processes in chemistry are those changes that proceed forward under given conditions. This page will help a student learn to recognize such processes.
What Does Spontaneous Mean?
 On the previous page of this lesson, we described Chemical Thermodynamics as the science of predicting and explaining whether or not a chemical or physical change will occur naturally, the direction in which it naturally occurs, and the extent to which it occurs. The general term given to a process that occurs naturally under a specific set of conditions is the term spontaneous. A spontaneous process is a process that occurs naturally on its own without any sustained or continued intervention.
On the previous page of this lesson, we described Chemical Thermodynamics as the science of predicting and explaining whether or not a chemical or physical change will occur naturally, the direction in which it naturally occurs, and the extent to which it occurs. The general term given to a process that occurs naturally under a specific set of conditions is the term spontaneous. A spontaneous process is a process that occurs naturally on its own without any sustained or continued intervention.
The rolling of a ball down a hill is an example of a spontaneous process. The ball may require a gentle push or nudge to get it started. But once started, the ball will continue rolling down a hill of any given length. Additional pushes or nudges will not be necessary to sustain the motion. It occurs naturally on its own without any continued external intervention.
Chemistry students are familiar with the combustion of methane gas in the Chemistry lab. Methane, the main component of natural gas, is often ignited as it exits a Bunsen burner during a chemistry lab. The ignition may occur by use of a lit match or a spark from a striker. Once ignited, the combustion of methane continues forever as long as there is a supply of methane and oxygen gas. The reaction is spontaneous and occurs naturally without requiring a continued intervention.
Conditions of Spontaneity
 Identifying a process as being spontaneous often assumes a set of temperature and pressure conditions. Combustion reactions are always spontaneous at 1 atm of pressure and room temperature. But there are conditions. First, they must be activated. Similar to the combustion of methane exiting the barrel of a Bunsen burner, a candle must first be lit to initiate or start the process. The activation is required to overcome the high activation energy and initiate the reaction. It is a one-time deal; it is not a continued intervention. Second, there must be a supply of reactants - the candle wax and oxygen. Provided the fuel and the oxygen are available and the reaction has been activated, the combustion of the fuel is considered spontaneous. Once activated, the process is naturally self-sustaining without the need for continued, external intervention.
Identifying a process as being spontaneous often assumes a set of temperature and pressure conditions. Combustion reactions are always spontaneous at 1 atm of pressure and room temperature. But there are conditions. First, they must be activated. Similar to the combustion of methane exiting the barrel of a Bunsen burner, a candle must first be lit to initiate or start the process. The activation is required to overcome the high activation energy and initiate the reaction. It is a one-time deal; it is not a continued intervention. Second, there must be a supply of reactants - the candle wax and oxygen. Provided the fuel and the oxygen are available and the reaction has been activated, the combustion of the fuel is considered spontaneous. Once activated, the process is naturally self-sustaining without the need for continued, external intervention.
The Direction of Spontaneity
A process that occurs spontaneously in one direction is not spontaneous in the opposite direction under the same conditions. An ice cube melts at room temperature. That is to say that the change of state described by the equation
H2O(s) → H2O(l) (25°C)
is spontaneous at ~25°C. However, at the same temperature, water in the liquid state does not change to ice at room temperature.
Similarly, liquid water will freeze at -10°C. However, it will not melt at -10°C. The freezing of water is spontaneous at temperatures below 0°C while the reverse process (melting) is not spontaneous under the same conditions.
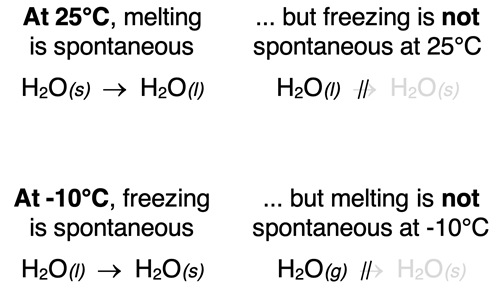
Examples of Spontaneous Processes
There are endless examples of spontaneous and non-spontaneous reactions. We have already mentioned a ball rolling down a hill, the combution of methane from a Bunsen burner, and the burning of a candle as spontaneous processes. Here are a few more.
The dissolving of sodium chloride in water is an example of a spontaneous process. As long the NaCl crystals and the water are in contact with each other, the water molecules will solvate the ions and dissolving will occur. The process will continue without intervention until the solution is saturated with sodium chloride. There are interventions that can be taken to increase the rate at which the salt dissolves - stirring, increasing the temperature of the water - but the rate at which a process occurs has nothing to do with it being spontaneous or non-spontaneous. The process of dissolving would continue on its own without these interventions.
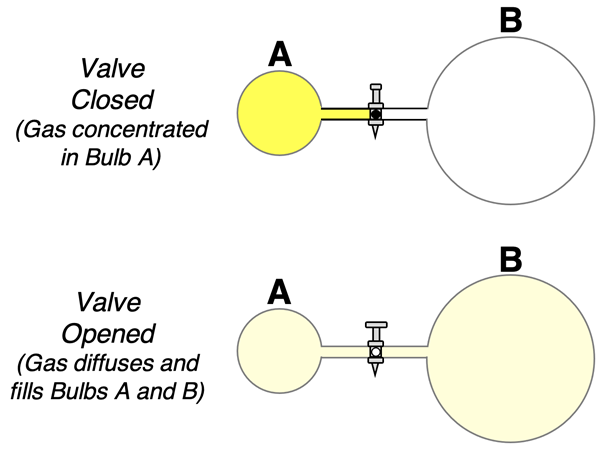
The diffusion of a gas is another example of a spontaneous process. Diffusion involves the spread of a gas from a location where it is concentrated to the surrounding space. Consider a two-bulb system separated by a valve like that shown below, with all the gas initially present in the Bulb A. If the valve is opened, some gas from Bulb A will diffuse to Bulb B. This occurs spontaneously and does not require a fan or the input of energy.
 The rusting of iron is our final example of a spontaneous process. Rusting involves the reaction between an iron surface and oxygen gas in a moist environment to produce flakes of hydrated iron(III) oxide. The process takes time but occurs naturally. It does not require the continuous input of energy or intervention from external influences. Steel is an alloy of iron and despite efforts to protect it from rust by the addition of additives, steel still rusts. Steel tools are particularly susceptible to rust over the course of time, as shown in the photo at the right.
The rusting of iron is our final example of a spontaneous process. Rusting involves the reaction between an iron surface and oxygen gas in a moist environment to produce flakes of hydrated iron(III) oxide. The process takes time but occurs naturally. It does not require the continuous input of energy or intervention from external influences. Steel is an alloy of iron and despite efforts to protect it from rust by the addition of additives, steel still rusts. Steel tools are particularly susceptible to rust over the course of time, as shown in the photo at the right.
Examples of Non-Spontaneous Processes
A non-spontaneous process is one that does not occur naturally on its own. A constant input of energy is often required for a non-spontaneous process to occur. A non-spontaneous process is akin to riding a bicycle up a hill. The process can occur, but it requires the continuous input of energy since the uphill ascent is not sustainable on its own.

Photosynthesis is an example of a non-spontaneous process. The process involves the reaction of carbon dioxide with water to produce glucose and oxygen. The process does not take place without sunlight.
The charging of a battery is another example of a non-spontaneous process. Your phone battery won’t charge if you fail to plug it in to the charger. The continued supply of energy from the charger to the phone is required to charge the phone battery.
Some processes are spontaneous at one set of pressure-temperature conditions but non-spontaneous at other conditions. Liquid water freezes at temperatures below 0°C but does not freeze at temperatures above the freezing point of 0°C.
Can an Endothermic Process by Spontaneous?
The short answer to the question is YES! The long answer to the question is in discussion of Gibbs Free Energy found in Lesson 3. For now, we will elaborate on the short answer.
Spontaneous processes are those that occur naturally on their own without external intervention. Let’s consider two processes - the melting of an ice cube at room temperature and the photosynthesis in plants. Both processes are endothermic. The enthalpy of the products are greater than the enthalpy of the reactants. Both processes climb up the enthalpy hill. But the melting of an ice cube at room temperature occurs naturally on its own without intervention. Plant photosynthesis is not spontaneous; it does not occur naturally on its own. What’s the distinction?
An ice cube melts naturally on its own. The flow of energy from the surroundings to the ice cube occurs as warmer air particles collide with the colder ice cube particles. This is a natural process; we refer to it as heat transfer. There is no way to stop or start it. It is an inescapable, passive event that occurs naturally between two objects of different temperatures.
Photosynthesis is also endothermic. But the nature of the energy input is quite different than the passive flow of energy that occurs as heat transfer. Photosynthesis does not occur because of heat flow into the plan. Instead, the energy input must be actively supplied by an external source - the sun or a grow light - that illuminate the plant with photons (light particles). The plant is unable to naturally turn the sun or grow light on or off. The “on its own” feature is absent in photosynthesis. Intervention is necessary and the process is classified as non-spontaneous.
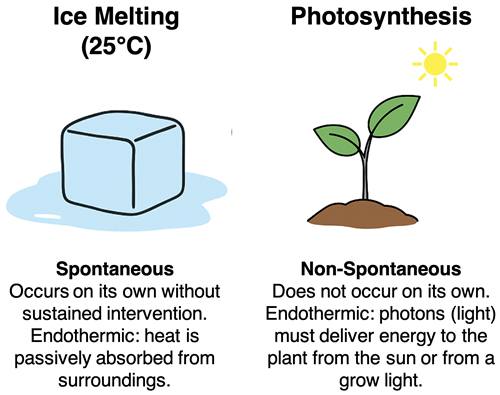
It’s easy to mix up the terms endothermic and non-spontaneous because both seem to involve energy going into something. But these two terms describe very different ideas. Endothermic describes the direction that energy flows relative to the system and surroundings. Spontaneous and non-spontaneous describe whether or not a process occurs naturally on its own without intervention. So, every non-spontaneous process has an energy input requirement; but not every process that absorbs energy is non-spontaneous. Ice melting and plant photosynthesis highlight this distinction.
Explanations of Spontaneity
The interesting questions (and answers) are the why questions. Exactly why does the melting of ice occur at 25°C but the opposite process - freezing - not occur under the same conditions? There are reasons and we will get to them. But we have some additional concepts to develop. By the end of Lesson 2, you will be able to provide conceptual explanations for spontaneity. And by the end of Lesson 3, you will be able to quantify those explanations. But before we start Lessons 2 and 3, we have to discuss the first law of thermodynamics. That’s our next page. Before plowing ahead, take some time to internalize the ideas presented here on spontaneity.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Try our Concept Builder titled Spontaneity and Driving Forces. The first of the three activities is a great follow-up to this lesson.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Spontaneous Processes. Save it to a safe location and use it as a review tool.
Check Your Understanding of Spontaneous Processes
Use the following questions to assess your understanding the meaning of spontaneous and non-spontaneous. Tap the
Check Answer buttons when ready.
1. Anna Litical and Aaron Agin live in a northern climate that experiences relatively extreme winter weather. They’re talking about their spontaneity lesson during lunch. Aaron is struggling with the claim that the melting of ice is not spontaneous below 0°C. His claim is that he’s seen icicles on his home melting on a sunny day when the temperature is -5°C. Anna has an answer for Aaron. But before you read it, make an attempt at an explanation of why ice melting at -5°C is not spontaneous despite the accurate observation of Aaron’s.
2. Identify the following statements as being
TRUE or
FALSE. For any
FALSE statement, correct the statement or prepare an explanation for why it is
FALSE.
- All spontaneous reactions are explosive reactions.
- All spontaneous reactions, like combustion reactions, release a large amount of energy.
- Spontaneous reactions occur very rapidly.
- If a truly spontaneous reaction is occurring, it will sustain itself without continuous intervention as long as it does not run out of one or more reactants.
- Sugar wont dissolve in room-temperature water unless it is stirred. Therefore, the dissolving of sugar in water is not spontaneous.
- Homework (the paper variety) won’t burn unless you light it. Since the burning of paper requires intervention, it is not a spontaneous process.
- Homework does not get completed unless you put sustained energy into its completion. Therefore, homework completion is not a spontaneous process.
3. Can a non-spontaneous process ever occur?
4. Identify the following processes as being spontaneous (
S) or non-spontaneous (
NS) under the conditions that are provided.
- Mrs. Mandocino has acquired a vessel full of liquid nitrogen for some demonstrations. Nitrogen has a boiling point of -196°C. She pours some of the liquid nitrogen onto the floor and it vaporizes.
- After several prompts, a few commands, and a final threat, Randy Mizashun violates his moral principles and decides to clean his room. To the surprise of his parents, he puts everything in its designated place.
- Cameron Per is (his friends call him Cam) is on a camping outing with his friends. After some hot dogs and several helpings of baked beans for dinner and some fireside chatting, they finally crowd into their tent like sardines in a can. Unfortunately for his friends, the beans are not settling well for Cam. He flatulates. The gas then fills the entire volume of the tent.
- In a classroom demonstration, Mr. Lebrum drops a couple of small chunks of calcium carbide in water. It immediately reacts to produce acetylene gas and calcium hydroxide.
- On their Physics field trip to Great America, a group of students are loaded into the roller coaster cars of the Batman ride. They are pulled from the loading station to the top of the tallest point on the track.
- After the ascent up the first hill is over, the coaster car begins its path around the turns, drops, hills, and loops for a 45-second thrill ride.
- Mr. Tooker planted several sugar snap peas in his backyard garden. They grew to 5 feet tall within six weeks.
- A large pump at the bottom of the water slide at the water park is used to pump water from the ground to the top of the hill.
- Suzie Lavtaski glides effortlessly down the ski hill to the ski lodge at its base.
- The freezing point of mercury is -38°C. The mercury in a glass thermometer freezes at 0°C.