Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Spontaneity and Energy
Part a: What is Thermodynamics?
Part a: What is Thermodynamics?
Part b:
Spontaneous Processes
Part c:
The First Law of Thermodynamics
The Big Idea
Thermodynamics is the branch of science that helps us answer: Which chemical changes are possible, in which direction do they proceed, and how far will they proceed?
Defining Thermodynamics
 Thermodynamics is a broad field of science that studies the relationship and interactions between the various forms of energy. A focus is placed upon how energy is transferred between objects and locations and how it is changes form. In Chemistry, thermodynamic concepts of energy and entropy are used to predict and explain whether or not a chemical or physical change will naturally occur, the direction in which it naturally occurs, and the extent to which it naturally occurs.
Thermodynamics is a broad field of science that studies the relationship and interactions between the various forms of energy. A focus is placed upon how energy is transferred between objects and locations and how it is changes form. In Chemistry, thermodynamic concepts of energy and entropy are used to predict and explain whether or not a chemical or physical change will naturally occur, the direction in which it naturally occurs, and the extent to which it naturally occurs.
Thermochemistry as a Branch of Thermodynamics
 The topic of Chapter 12 of our Chemistry Tutorial was Thermochemistry. Thermochemistry is a branch of the broader topic of thermodynamics. In our Thermochemistry chapter, we focused on the energy changes associated with chemical reactions and physical changes. Frequent attention was given to how energy transfers to or from the surroundings and the relation between this energy and the breaking and forming of bonds during chemical reactions. A large part of that discussion dealt with the concept of enthalpy and enthalpy changes associated with chemical reactions. We learned that enthalpy accounts for the total energy of a chemical system. For a reaction occurring at a constant pressure, the enthalpy change of the reaction is equal to the quantity of heat transferred between the chemical system and the surroundings. The concept of enthalpy will resurface in this chapter.
The topic of Chapter 12 of our Chemistry Tutorial was Thermochemistry. Thermochemistry is a branch of the broader topic of thermodynamics. In our Thermochemistry chapter, we focused on the energy changes associated with chemical reactions and physical changes. Frequent attention was given to how energy transfers to or from the surroundings and the relation between this energy and the breaking and forming of bonds during chemical reactions. A large part of that discussion dealt with the concept of enthalpy and enthalpy changes associated with chemical reactions. We learned that enthalpy accounts for the total energy of a chemical system. For a reaction occurring at a constant pressure, the enthalpy change of the reaction is equal to the quantity of heat transferred between the chemical system and the surroundings. The concept of enthalpy will resurface in this chapter.
System and Surroundings
A prevalent model for the study of both thermochemistry and thermodynamics involves the designation of a system and surroundings. The system is typically defined as the part of the universe that is being studied. In Chemistry, we typically think of the reactant and product chemicals as being the system. That is why we often refer to it as the chemical system. The surroundings is the rest of the universe. Together, the system and the surroundings make up all that there is - the universe.
Changes occur within the system. These changes involve changes of reactants to products. And as a consequence of these changes, there is also a change in energy or enthalpy. In chemistry, we typically do not regard the system as being closed off from the rest of the universe; there is a flow of energy in the form of heat from the system to the surroundings or from the surroundings to the system. We can observe and even measure changes in the temperature of the surroundings and draw some conclusions regarding the energy changes occurring within the system.
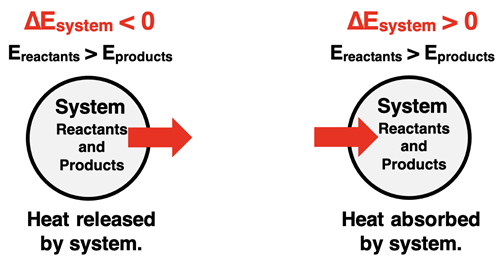
This system-surroundings model or way of thinking about chemical and physical change was introduced and used in our Thermochemistry Chapter. We will rely upon the same model in this chapter on Thermodynamics.
Thermodynamic Laws and State Functions
There are four recognized laws of thermodynamics. We will give considerable attention to the first and the second laws of thermodynamics. These laws were not received on stone tablets and there is typically no single set of words for stating any one of the laws. One textbook’s statement of the first law of thermodynamics will differ from that of another textbooks; and that really is not important. How the law is stated does not matter, as long as it is stated properly. How a student makes meaning of the law does matter. We will have our own statement of the first law (and it won’t differ much from any other statement); but our emphasis will be on making sense of what it means ... particularly, what it means in the context of chemistry and chemical reactions.
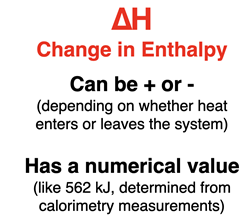
Much of our discussion of these laws will refer to the so-called state functions of enthalpy, entropy, and free energy. A state function is a property of a system whose value is dependent upon the current state of the system and not on the path by which that state was reached.
Enthalpy is an example of a state function. The enthalpy of 1 mole of H2O at 25°C and 1 atm is always the same regardless of how this state is obtained. Consider the following ways to obtain 1 mole of H2O at 25°C and 1 atm.
- Start with 1 mole of H2O at 50°C and 1 atm; allow it to cool to 25°C.
- Start with 1 mole of H2O at -5°C and 1 atm; melt it and then warm it up to 25°C.
- Start with 1 mole of H2 and 0.5 mole of O2 and react them to produce 1 mole of H2O at 25°C and 1 atm
These three statements describe three different pathways to obtaining 1 mole of H
2O at 25°C and 1 atm. The enthalpy of water in this final state will be the same regardless of the path. Enthalpy is a state function; as such, its value depends only on the state and not on how that state was reached.
Our model for thinking about state functions is to consider how they change when the system changes from an initial state to a final state. A typical change in a chemical system would be:
Initial State: 1 mole of N2(g) and 3 moles of H2(g) at 1 atm and 25°C.
Final State: 2 moles of NH3(g) at 1 atm and 25°C.
The change in enthalpy - ∆H - associated with this change is the difference in enthalpy between the initial and the final state. The change can be positive or negative, corresponding to either an increase or a decrease in the enthalpy of the system. Our model will involve thinking about changes in these state variables in terms of direction (increasing or decreasing) and in terms of numerical value (for instance, 562 kilojoule).
Thermodynamics vs. Other Models
A great deal of chemistry pertains to understanding chemical reactions. To that end, our
Chemistry Tutorial has introduced a variety of models for thinking about different aspects of chemical reactions. Here’s a quick summary of those models with a brief description of each.
- Stoichiometry: conservation of mass and the mathematical relationship between the amount of reactants and products.
- Thermochemistry: a study of the energy changes associated with chemical reactions.
- Kinetics: a study of the rates at which reactions occur, the factors affecting those rates, and of the pathway between reactants and products.
- Equilibrium: a study of the extent to which a reaction occurs and of how reactions respond to changes in temperature, pressure, and the addition of reactants or products.
In this chapter, we will add a fifth model:
- Chemical Thermodynamics: a study of whether a reaction occurs naturally, the direction in which the reaction naturally occurs (forward vs. reverse), and the extent to which the reaction occurs.
Each of these models has a unique set of rules for thinking about reactions. These ways of thinking allow chemists (and chemistry students) to answer a variety of questions about chemical reactions. But each model has its own
lane. While many of the models have some points of connection, it is important to use the appropriate model for the specific question. For instance, thermodynamics can predict whether or not a reaction would naturally occur, but it cannot predict the rate at which it occurs. Only
kinetics can predict a reaction rate. Thermodynamics predicts that diamond will change spontaneously into graphite but it cannot predict the rate at which the change occurs. Meanwhile, kinetics predicts that the change of diamond to graphite occurs very, very slowly, lending credence to the popular claim that
diamonds are forever.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on What is Thermodynamics? Save it to a safe location and use it as a review tool.
Check Your Understanding of What is Thermodynamics?
Use the following questions to assess your understanding of chemical thermodynamics involves. Tap the Check Answer buttons when ready.
1. Based on the given description of chemical thermodynamics, which of the following claims would emerge from the application of thermodynamic principles? Select all that apply.
- At 120°C and 1.00 atm, the reaction occurs to completion.
- The reaction only takes place at temperatures below 43°C.
- The reaction occurs very rapidly.
- The enthalpy change is positive and the entropy change is negative.
- The limiting reactant is nitrogen gas.
2. Identify the name of the three state functions mentioned in this lesson.
3. Describe how a state function is different than a path function (the opposite of a state function).
4. This lesson described five
lanes of chemistry (stoichiometry, thermochemistry, kinetics, equilibrium, and thermodynamics) concerned with chemical reactions. Match each one of the five questions with the lane that is best equipped to answer the question.
- Is the reaction endothermic or exothermic?
- How does an increases in reactant concentration affect the rate at which the reaction occurs?
- How can it be explained that ice does not melt at a temperature of -20°C?
- What is the limiting reactant when 3.0 g of H2 are combined with 32.0 g of O2 in the water synthesis reaction?
- How does the removal of ammonia from the system alter the concentrations of reactants and products?