Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: A Model of Solutions
Part d: Solubility, Temperature, and Pressure
Part a:
What is a Solution?
Part b:
Solubility and Structure
Part c:
The Dissolving Process
Part d: Solubility, Temperature, and Pressure
Part e:
Dissociation of Ionic Compounds
The Big Idea
The solubility of a substance doesn’t depend only on what the substance is. Solubility also depends on the temperature and pressure. This lesson explores how these two variables affect the solubility of solids and gases in water.
Effect of Temperature on the Solubility of Ionic Compounds
Solubility refers to the maximum amount of solute that can be dissolved in a specified amount of solvent. The actual solubility value depends on the solute and the solvent. But it also depends upon temperature. For ionic solids or salts, the solubility in water is most often observed to increase with increasing temperature. But there are other salts whose solubility in water is observed to decrease with increasing temperature.
The explanation of temperature effects on salt solubility in water usually relies on LeChatelier’s Principle - a principle to be discussed in Chapter 15 of this Chemistry Tutorial. We will save that explanation for Chapter 15. For now, we will use a particle level explanation.
As discussed in Lesson 1c, dissolving a salt in water involves two steps:
- Ions are pulled away from the crystal lattice (an endothermic step related to lattice energy).
- Separated ions are attracted to and surrounded by water molecules (an exothermic step related to solvation energy or hydration energy).
For many salts, the lattice energy (step 1) is so great that the overall dissolving process is endothermic. These salts are the ones whose solubility is typically increased by increasing temperatures. At higher temperatures, water molecules move about the container with greater speeds and kinetic energy. These increased speeds allow water to be more effective at penetrating the surface of the crystal and pulling ions away from the lattice and into the solution. And with a greater speed, water molecules can pull the solvated ion away from the crystal more quickly and reduce the likelihood of recrystallization.
Some salts are less soluble in water at higher temperatures. This is most commonly observed of salts with such a large solvation energy (step 2) that the overall dissolving process is exothermic. For such salts, the higher speeds of water molecules at higher temperatures makes them less able to keep the solvated ions stabilized. Solvated ions are more likely to escape water’s hold on them and recrystallize. This lowers the solubility at higher temperatures.
Solubility Curves
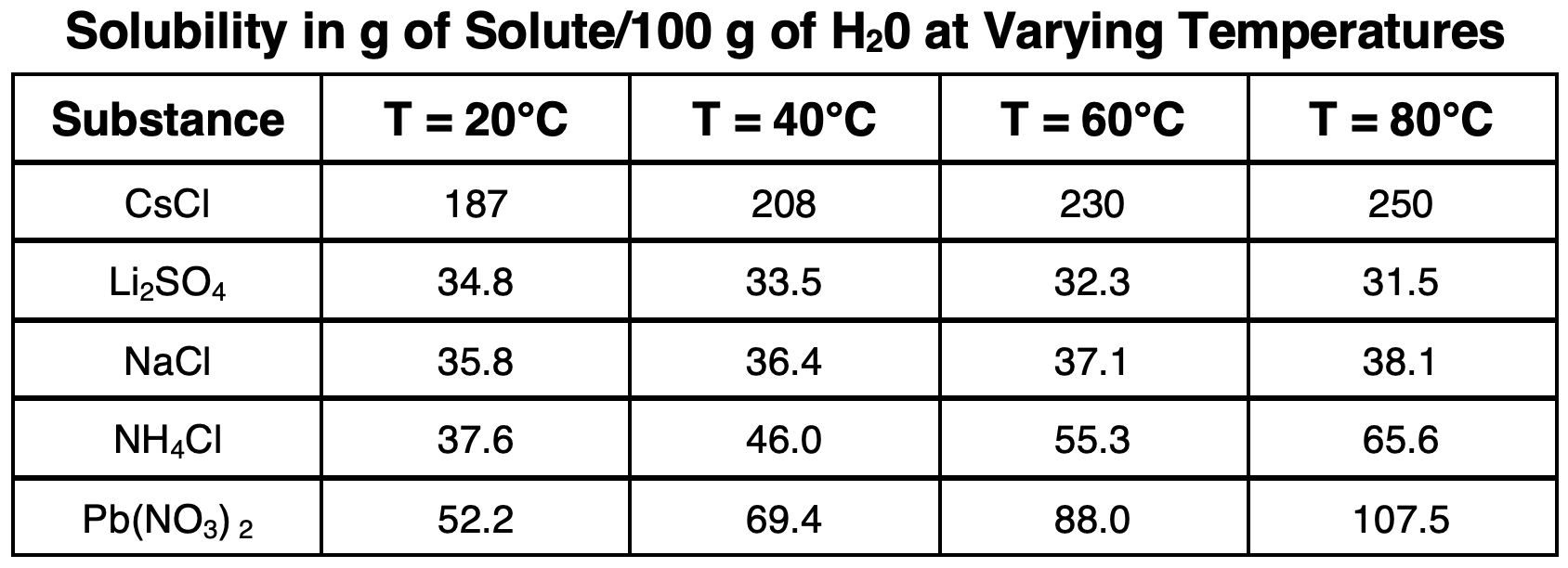
The graph below represents the solubility of varying ionic solids for a range of temperatures from 0°C to 100°C. Each line is referred to as a
solubility curve. The units on solubility are
grams of solute/100 g of water.

Observe how most lines on the graph slope or curve upward, indicating an increase in solubility with increasing temperature. Two substances - Li
2SO
4 and SO
2 slope downward. Sulfur dioxide is a gas and follows the usual trend for gases - decreasing solubility with increasing temperatures. Lithium sulfate (Li
2SO
4) is an ionic solid that is less soluble at higher temperatures. There are other salts (not shown) that follow the same trend as Li
2SO
4.
Saturated vs. Unsaturated Solutions
Let’s consider the ammonium chloride (NH
4Cl) line on the chart. At 30°C, approximately 40.0 g of NH
4Cl can dissolve in 100.0 g of water. This is the limit for solubility for this solute at this temperature. An NH
4Cl
(aq) solution that contains 40.0 g NH
4Cl for every 100.0 g of H
2O is said to be a
saturated solution. Additonal solute cannot be dissolved in the water at this temperature. If a NH
4Cl
(aq) solution contains less than 40.0 g NH
4Cl for every 100.0 g of H
2O, then it is said to be an
unsaturated solution.
Suppose that 50.0 g NH
4Cl were added to a 100.0 g of H
2O at 30.0°C and stirred. What would happen? Using the solubility of NH
4Cl from
the solubility chart, we can predict that 40.0 g NH
4Cl will dissolve in the solution and the remaining 10.0 g NH
4Cl will remain undissolved. No amount of stirring or waiting or wishing will dissolve those additional 10.0 g NH
4Cl. The solution is satured with NH
4Cl once the 40.0 g NH
4Cl is dissolved.
Now suppose that 25.0 g NH
4Cl were added to a 100.0 g of H
2O at 30.0°C and stirred. What would happen? All the NH
4Cl would dissolve in the water. There would be no undissolved NH
4Cl present in the container. The solution would be unsaturated, meaning additional NH
4Cl could be added and dissolved. In fact, using the solubility of NH
4Cl from
the chart, we can predict that an additional 15.0 g of NH
4Cl could be added and dissolved in the solution.
Interpreting Solubility Curves
Solubility values are ratios - ratios of the mass of solute per mass of solvent (H
2O). The solution doesn’t always contain 100.0 g water, but the ratio of mass of solute per mass of solvent applies to all solutions regardless of the mass of solvent. Let’s consider an aqueous solution of NH
4Cl at 30.0 °C. The solubility of NH
4Cl is 40.0 g/100.0 g H
2O. What maximum amount of NH
4Cl can be dissolved into 50.0 g H
2O? What maximum amount of NH
4Cl can be dissolved into 200.0 g H
2O? For both questions, we can solve by using a ratio (or a conversion factor).
The following examples illustrate how the solubility curves can be used to make predictions. Use the
Solubility Curves to determine the answers. Then check your answers.
Example 1 - Saturated or Unsaturated?
Use the graph of solubility curves to determine if the following solutions would be saturated or unsaturated with solute under the given conditions.
- A solution is made by adding 60.0 g of NaNO3 to 100.0 g of H2O at 20°C.
- A solution is made by adding 100.0 g of NaNO3 to 100.0 g of H2O at 40°C.
- A solution is made by adding 60.0 g of NaNO3 to 50.0 g of H2O at 20°C.
- A solution is made by adding 100.0 g of NaNO3 to 200.0 g of H2O at 40°C.
- A solution is made by adding 60.0 g of NaNO3 to 50.0 g of H2O at 60°C.
Example 1 - Answers and Explanations:
All five parts of this question involve the same solute (NaNO
3). Three different temperatures are involved.
The solubility curve for NaNO3 can be used to estimate the solubility at these three temperatures:
- At 20°C, solubility = ~86 g NaNO3/100.0 g H2O.
- At 40°C, solubility = ~100 g NaNO3/100.0 g H2O.
- At 60°C, solubility = ~120 g NaNO3/100.0 g H2O.
Now the information can be evaluated to answer Parts a-e. If the ratio of NaNO
3 to H
2O is less than the solubility, the solution is unsaturated. If the ratio is equal to the solubility, then the solution is saturated. And if the ratio is greater than the solubility, the solution is still saturated but there will be undissolved NaNO
3 remaining at the bottom of the container.
Answers:
- Unsaturated (an additional 26 g NaNO3 could be dissolved in the water)
- Saturated
- Saturated (with 17 g of undissolved NaNO3 at the bottom of the container)
- Unsaturated (200 g H2O can hold up to 200 g NaNO3 at this temperature)
- Saturated (the ratio of NaNO3 to H2O is equivalent to the solubility of 120:100)
Example 2 - Determining the Mass of Solute that can Dissolve
For each situation below, use the graph of solubility curves to determine the maximum amount of solute that can be dissolved in the given amount of H
2O at the indicated temperature.
- How much LiCl can be added to 100.0 g of water at 20°C?
- How much LiCl can be added to 200.0 g of water at 20°C?
- How much LiCl can be added to 100.0 g of water at 80°C?
- How much NH4Cl can be added to 100.0 g of water at 90°C?
- How much NH4Cl can be added to 50.0 g of water at 90°C?
Example 2 - Answers and Explanations:
The process will involve
looking up the solubility for the given solute at the given temperature. Then use the solubility as part of a ratio to determine the amount of solute that must be added.
- Solubility = ~80 g LiCl/100.0 g H2O. So, X/100.0 g H2O = 80 g LiCl/100.0 g H2O
Solving for X, we get 80 g LiCl.
- Solubility = ~80 g LiCl/100.0 g H2O. So, X/200.0 g H2O = 80 g LiCl/100.0 g H2O
Solving for X, we get 160 g LiCl.
- Solubility = 102 g LiCl/100.0 g H2O. So, X/100.0 g H2O = 102 g LiCl/100.0 g H2O
Solving for X, we get 102 g LiCl.
- Solubility = ~70 g NH4Cl/100.0 g H2O. So, X/100.0 g H2O = 70 g NH4Cl/100.0 g H2O
Solving for X, we get 70 g NH4Cl.
- Solubility = ~70 g NH4Cl/100.0 g H2O. So, X/50.0 g H2O = 70 g NH4Cl/100.0 g H2O
Solving for X, we get 35 g NH4Cl.
Example 3 - How Much More Solute can Dissolve?
For the following descriptions of an unsaturated solution,
use the graph of solubility curves to determine the amount of additional solute that can be added to the solution before it becomes saturated.
- A solution is made at 30°C by adding 20.0 g of NaC2H3O2 to 100.0 g of water. How much additional NaC2H3O2 can be added before the solution is saturated?
- A solution is made at 50°C by adding 40.0 g of NaC2H3O2 to 100.0 g of water. How much additional NaC2H3O2 can be added before the solution is saturated?
- A solution is made at 50°C by adding 40.0 g of NaC2H3O2 to 50.0 g of water. How much additional NaC2H3O2 can be added before the solution is saturated?
Example 3 - Answers and Explanations:
The solution begins by determining the solubility for the given temperature. This will provide the ratio of NaC
2H
3O
2 to H
2O that is required for a saturated solution. From this, you can determine the total mass of NaC
2H
3O
2 that would be required for the given volume of H
2O. Then the additional mass that must be added to reach this total can be determined.
- Solubility = ~50 g NaC2H3O2/100.0 g H2O. Since the solution contains 100.0 g of H2O, there will need to be a total of 50 g NaC2H3O2 to saturate it. Thus, 30 g NaC2H3O2 should be added to the 20.0 g that are already present.
- Solubility = ~90 g NaC2H3O2/100.0 g H2O. Since the solution contains 100.0 g of H2O, there will need to be a total of 90 g NaC2H3O2 to saturate it. Thus, 50 g NaC2H3O2 should be added to the 40.0 g that are already present.
- Solubility = ~90 g NaC2H3O2/100.0 g H2O. Since the solution contains 50.0 g of H2O (one-half of 100 g), there will need to be a total of 45 g NaC2H3O2 (one-half of 90 g) to saturate it. Thus, 5 g NaC2H3O2 should be added to the 40.0 g that are already present.
What is a Supersaturated Solution?

From what has been said so far, it may be surprising to learn that a solution can be supersaturated. A
supersaturated solution is a solution that contains more than the maximum amount of solute. A supersaturated solution cannot be made by simply adding more than the limit of how much solute the solvent can contain. Supersaturated solutions are made by taking advantage of
the solubility-temperature relationship. For many salts, the solubility is greater at a higher temperature. A saturated solution at a high temperature will become a supersaturated solution when cooled down to a lower temperature. Let’s once again consider aqueous ammonium chloride - NH
4Cl
(aq) - to illustrate the concept.
At 90°C, the solubility of NH
4Cl is 70.0 g per 100.0 g H
2O. Suppose we heated a beaker of 100.0 g of H
2O to a temperature of 90°C. Then we stirred in and dissolved 70.0 g of NH
4Cl to saturate it. Now suppose we left the beaker of saturated NH
4Cl
(aq) on the lab bench and allowed it to cool down to a temperature of 30°C. What would happen? Nothing … other than the temperature changed by 60°C … and the solubility changed from 70 g/100.0 g H
2O. to 40.0 g/100.0 g H
2O. At 30°C, our beaker still contains 70.0 g NH
4Cl. But this is 30.0 g above the limit at the new temperature. The solution is a supersaturated solution.
The solubility curve below shows graphically how the supersaturated solution was made.
- Beginning at 90°C, a saturated solution was made by adding 70.0 g NH4Cl to 100.0 g H2O.
- The solution was then allowed to cool to 30°C where the solubility is less - 40.0 g NH4Cl per 100.0 g H2O.
- Because there was no change in the amount of solute or solvent in the beaker, the solution is now supersaturated, containing an extra 30.0 g of NH4Cl(aq).

A supersaturated solution is
not a stable solution. It is a solution that is
bloated with dissolved solute that is ready to drop out of the solution. The dissolved ions are surrounded by water, postponing their ability to attract and form a crystalline precipitate. To initialize precipitation of the extra, above-the-limit solute, a crystal of the solid can be dropped into the solution. The crystal provides a site about which precipitation can occur. Solvated ions escape and attach to the crystal. Precipitation rapidly accelerates and spreads through the entire container. Bumping or disturbing the container can also cause the excess solute to precipitate.
Photo Source:
Wikimedia Commons
Example 4 - Making a Supersaturated Solution
A beaker containing 100.0 g of H
2O is heated to 80°C. Sodium acetate (NaC
2H
3O
2) is then dissolved into the solution to saturate it.
- How many grams of NaC2H3O2 should be added to the 100.0 g of H2O to saturate the solution?
- Once the solution is made, it is set aside to cool to 30°C. How many grams of NaC2H3O2 are present in the solution once it has cooled to 30°C?
- What is the solubility of NaC2H3O2 at 30°C? Is the solution unsaturated, saturated, or supersaturated?
- Describe what will happen if a small crystal of NaC2H3O2 is added to the solution. Be quantitative in your description.
Example 4 - Answers and Explanations:
- At 80°C, the solubility is ~150 g NaC2H3O2/100.0 g H2O. Add and dissolve 150 g NaC2H3O2 in the water.
- There will still be 150 g NaC2H3O2 dissolved in the water. Cooling the solution does not change the amount of solute or solvent present.
- At 30°C, the solubility is ~50 g NaC2H3O2/100.0 g H2O. The solution is supersaturated since it contains more than 50 g of NaC2H3O2.
- A supersaturated solution is unstable and will precipitate some of the excess solute when a crystal is added. So, adding the crystal will cause 100.0 g NaC2H3O2 to precipitate. This will leave 50.0 g NaC2H3O2 dissolved in the 100.0 g of H2O.
Effect of Temperature on Gas Solubility
Unlike many solids, the solubility of gases in water are less at higher temperature. The inverse relationship between gas solubility and temperature is shown in the graph below.
Like solids, a gas particle must be attracted and surrounded by water molecules to become part of the aqueous solution. At higher temperatures, gas particles that have entered the water are moving faster and are more difficult to surround. At higher temperatures, they are more likely to escape the liquid before being surrounded by water.
Effect of Pressure on Gas Solubility
A final factor that affects the solubility of a gas in water is the pressure of the gas above the solution. At higher pressures, the concentration of gas particles in the space above the water increases. This increases the frequency at which gas particles collide with the water surface. Increases in those collisions provides more opportunities for water molecules to attract, surround, and stabilize the gas particles in the aqueous state.
In 1803, English chemist William Henry proposed an equation relating the solubility of a gas in a solvent to the pressure of the gas above the solvent. Henry proposed that the concentration of a gaseous solute dissolved in a liquid solvent is proportional to the pressure of the gas above the solvent. His equation, now known as
Henry’s Law, is
C = k•P
where
C is the concentration of the solute in the solution,
P is the pressure of the gas above the solution, and
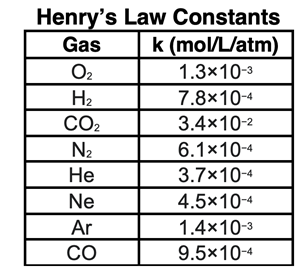
k is a constant of proportionality that depends upon the identities of the solute and the solvent. Values of k, referred to as Henry’s Law constants, are determined experimentally. The table at the right shows a sampling of k values for a variety of gases dissolving in water at a temperature of 25°C.
Example 5 - Henry's Law Calculations
Determine the concentration of CO
2 gas at 25°C in mol/L and g/L in an aqueous solution at a pressure of …
- 1.0 atm.
- 2.0 atm.
- 4.0 atm.
- 10.0 atm.
Solution:
Henry’s Law constant (k) for CO
2 in water is 3.4x10
-2 mol/L/atm or 0.034 mol/L/atm. The concentration in mol/L can be calculated for the Part a - d pressures using the equation C = k•P. Once determined in mol/L, the molar mass of CO
2 (44.01 g/mol) can be used to determine the concentration in g/L. The two-part answers for a.-d. are shown below:
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding of Temperature, Pressure, and Solubility
Use the following questions to assess your understanding of the effect of temperature and pressure upon solubility. Tap the Check Answer buttons when ready.
1. Identify the following statements as being True or False. If False, correct the statement or explain what is wrong with it.
- A large lattice energy is likely to cause a dissolving process a exothermic process.
- All ionic solids are soluble in water.
- Increasing temperature always increases the solubility of a salt in water.
- Increasing temperature always decreases the solubility of a gas in water.
- If a salt has a solubility of 20 g/100 g H2O at a temperature of 25°C, then a supersaturated solution can be made by adding 30 g of solute to 100 g H2O at 25°C.
- Increasing the pressure of a gas above a liquid solvent will cause more gas to be dissolved in that solvent.
2. Consider the solubility curves for solutes X, Y, and Z in water.
- Determine the solubility of substance X at 20°C.
- Determine the solubility of substance Y at 40°C.
- Determine the solubility of substance Z at 60°C.
- Approximately how many times more soluble is substance X than substance Y at 20°C?
- Approximately how many times more soluble is substance X than substance Z at 50°C?
3. Consider the solubility curves for solutes X, Y, and Z in water.
- How many g of X can be dissolved in 100 g H2O at 50°C?
- How many g of X can be dissolved in 50 g H2O at 50°C?
- How many g of X can be dissolved in 200 g H2O at 50°C?
- How many g of Y can be dissolved in 100 g H2O at 70°C?
- How many g of Y can be dissolved in 80 g H2O at 70°C?
- An aqueous solution of Z contains 20 g of Z in 100 g of H2O at 40°C. What additional mass of Z must be added to the water for the solution to be saturated?
- An aqueous solution of Z contains 20 g of Z in 50 g of H2O at 80°C. What additional mass of Z must be added to the water for the solution to be saturated?
- Noah Formula adds 120 g of Y to 100 g H2O at 50°C and stirs for several minutes. What type of solution is formed - unsaturated, saturated, supersaturated? Is there any undissolved Y remaining in the beaker? If so, then how much?
4. Anna Litical is attempting to create a supersaturated solution of Y (referring to above graph). She starts with a beaker of 100.0 g of H2O at 90°C. Describe the steps she must perform to create a supersaturated solution of Y at 20°C.