Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: A Model of Solutions
Part c: The Dissolving Process
Part a:
What is a Solution?
Part b:
Solubility and Structure
Part c: The Dissolving Process
Part d:
Solubility, Temperature, and Pressure
Part e:
Dissociation of Ionic Compounds
The Big Idea
The dissolving process involves more than "stuff mixing". When a substance dissolves, its particles break free from one another and interact with solvent molecules. In this lesson, we'll examine the step-by-step process that governs how and why materials dissolve.
What Does Dissolving Involve?
In Lesson 1b, we learned the importance of intermolecular forces to the dissolving process. Dissolving a solid in water involves a state change. The solute changes from the solid state to the aqueous state. In the solid state, particles (ions, atoms, or molecules) are attracted to one another by intermolecular forces. These forces hold the particles in the solid state. Dissolving involves weakening those intermolecular forces and forming new attractions to the solvent molecules. For a solid to dissolve in water, its particles must exchange the intermolecular attractions that it has for one another for intermolecular attractions to water. In the aqueous state, the solute particles are attracted to water molecules by new intermolecular forces.

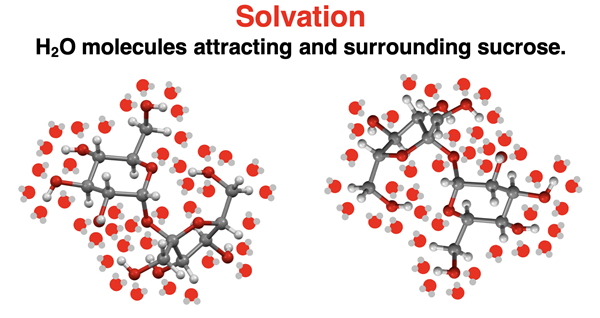
Solvation
At the heart of dissolving a solute is solvation. Solvation is the process in which solvent molecules surround and attract the solute particles. When the solvent is water, solvation is sometimes referred to as hydration. By surrounding the solute particles, the solvent prevents the particles from rejoining and returning to their non-dissolved state. Water is an effective solvent for ionic solids because of its polarity. Its positive pole (nearest the H atoms) can attract and surround the anions of the ionic compound. And its negative pole (nearest the O atom) can attract and surround the cations of the ionic compound. These attractive forces are referred to as ion-dipole interactions. Water is also an effective solvent of polar molecular compounds because of its ability to form strong dipole-dipole interactions (including hydrogen bonding) with the polar solute molecules. By surrounding and attracting the solute, water prevents the molecules from returning to its undissolved state. Finally, nonpolar solvents surround and attract nonpolar solutes through London dispersion forces.

 Dissolving Molecular Solids in Water
Dissolving Molecular Solids in Water
Perhaps you have poured a teaspoon (or more) of table sugar into a glass of water and stirred it. The sugar dissolves in the water and there is no evidence of any undissolved sugar at the bottom of the glass. The untrained mind might assert that “the sugar is gone.” But not you. You know that the sugar has simply changed state from (s) to (aq). And the best evidence to support your claim is that when you drink the water it has the taste of sugar. How does the sugar dissolve in the water?
 Table sugar is chemically known as sucrose (C12H22O11). It is a large polar covalent molecule with a carbon backbone and several O-H groups. Its molecular structure is shown at the right; the O-H groups are highlighted. The O-H groups of sucrose are credited for the hydrogen bonding forces that hold the molecules of sucrose in their crystal lattice.
Table sugar is chemically known as sucrose (C12H22O11). It is a large polar covalent molecule with a carbon backbone and several O-H groups. Its molecular structure is shown at the right; the O-H groups are highlighted. The O-H groups of sucrose are credited for the hydrogen bonding forces that hold the molecules of sucrose in their crystal lattice.
Water is also capable of hydrogen bonding, making it an effective solvent of sucrose. Water molecules are in random constant motion. They collide with the sucrose molecules on the exterior surface of the crystal and exert competing hydrogen bonding forces upon those surface molecules. One molecule at a time, the water pulls a sucrose molecule away from the crystal, attracts it with hydrogen bonds, and surrounds it so that it never returns to the crystal. Stirring speeds up the process by distributing sucrose crystals throughout the entirety of the container and allowing water access to the entire perimeter of the surface. Stirring also serves to move already solvated sucrose molecules away from the crystal’s surface, allowing other water molecules fresh access to undissolved sucrose molecules.
Dissolving Ionic Solids in Water
Ionic compounds do not consist of molecules, but of cations and anions held together in a crystal lattice by strong ionic bonds. Ionic bonds are considerably stronger than the intermolecular forces that holds molecular compounds together as a solid or liquid. Thus, not all ionic compounds dissolve in water. But for those that do, the process is always the same. The water attracts, separates, and surrounds the cations and anions and prevents them from returning to the lattice structure.
 An ionic compound is often referred to generically as a salt. When salts dissolve in water, the dissolved solute particles are cations and anions. Rather than solvating a molecule, the water must solvate individual ions. When the salt crystal enters water, the water molecules collide with the ions on the exterior of the crystal. Each collision results in an attractive ion-dipole interaction between the ions of the salt and the positive and negative poles of the polar water molecule. These interactions are stronger interactions than hydrogen bonding and serve to break the lattice apart and free the ions from the crystal. The ions are quickly surrounded by the water and prevented from returning to the crystal. The process produces two different aqueous-state ions. For sodium chloride dissolving in water, we would write:
An ionic compound is often referred to generically as a salt. When salts dissolve in water, the dissolved solute particles are cations and anions. Rather than solvating a molecule, the water must solvate individual ions. When the salt crystal enters water, the water molecules collide with the ions on the exterior of the crystal. Each collision results in an attractive ion-dipole interaction between the ions of the salt and the positive and negative poles of the polar water molecule. These interactions are stronger interactions than hydrogen bonding and serve to break the lattice apart and free the ions from the crystal. The ions are quickly surrounded by the water and prevented from returning to the crystal. The process produces two different aqueous-state ions. For sodium chloride dissolving in water, we would write:
NaCl(s) → Na+(aq) + Cl-(aq)
The above equation is referred to as a dissociation equation. We will discuss these equations in further detail in Lesson 1e.
Lattice Energy and Solvation Energy
The dissolving process involves two steps:
- Breaking a solute particle away from the lattice.
- Solvating the solute particle by solvent particles.
The first step is an
endothermic step. It requires energy to pull an ion or a molecular away from its crystal lattice. The energy required for this step is most closely related to the
lattice energy. The second step is an
exothermic step. Energy is released when intermolecular forces are formed between solute and solvent particles. The energy released by this step is most closely related to the
solvation energy. The magnitude of these two energies varies from solute to solute and from solvent to solvent.
The
heat of solution (∆H
soln) is the enthalpy change associated with dissolving 1 mole of a solute in a solvent. Values of the heat of solution are equal to the energy required to break apart the particles of the lattice plus the energy released when solvating those particles in the solvent. For ionic solids, most heat of solution values are positive, meaning the dissolving process is endothermic (though there are certainly numerous exceptions to the trend). Nature favors processes that are overall exothermic. Changes that raise the energy of a system are less likely to occur than those that decrease the energy. So why do such solids dissolve if the process is endothermic? In Chapter 16 of our
Chemistry Tutorial, we will learn about a second factor that affects the likelihood that a process occurs naturally. That factor is
entropy. For the dissolving of an ionic solid in water, entropy is always on the side of the process occurring. If the heat of solution is a relatively low positive value, then entropy will be the game-changer and dissolution will occur.
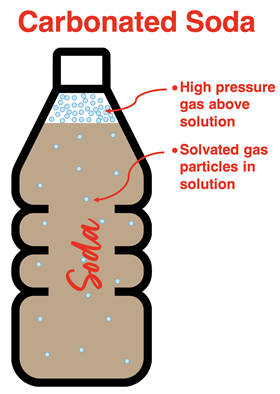
Solubility of Gases in Water
The dissolving of a gas in water follows a slightly different mechanism as the dissolving of a solid in water. First, it is not possible to drop a spatula-full of gas into water like can be done for a solid. And second, gases (the ideal gas variety) do not have intermolecular attractions for one another. They exist as separate particles and not in a condensed state. Thus, the water does not need to separate a gas particle from a lattice. But a strategy is still needed for coercing the gas to enter the water. Once the gas enters the water, solvation occurs in the usual manner through
intermolecular forces between water and the gaseous solute.
When gases are present in the space above water, they will collide with the water surface. A small percentage of these collisions result in a collection of water molecules attracting and solvating the gas particle. The frequency of collisions increases with an increasing concentration of the gas above the water. The most common method of increasing the solubility of a gas involves increasing the pressure (and thus, the concentration) of the gas particles. This results in more collisions and an increased solubility. Makers of carbonated beverages rely on increased pressures to increase the amount of carbon dioxide dissolved in the beverage. The can or bottle is carbonated at high pressure. The audible fizz that is heard when a new can is opened is the result of the pressure inside the can being suddenly released.
A second method of increasing the ease at which the gas can be solvated by water involves bubbling the gas through the water. This is the gaseous equivalent to dropping a spatula full of solid into water and stirring. By

bubbling the gas into the water, the number of interactions between molecules of water and particles of gas increases and the solution is more rapidly saturated with gas. Aerators are commonly used in ponds to increase the amount of interaction between water molecules and air in an effort to increase dissolved oxygen levels in the pond.
In
Lesson 1d, we will learn more about the effects of temperature and pressure upon the solubility of a solid and a gas in water. Before you head there, take some time to internalize the ideas of Lesson 1c using one of the ideas in the
Before You Leave section.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding the Dissolving Process
Use the following questions to assess your understanding of how solutes dissolve in water. Tap the Check Answer buttons when ready.
1. Describe the meaning of solvation in your own words.
2. What pole of water’s dipole is attracted to a cation during the solvation process? And what pole of water’s dipole is attracted to a anion during the solvation process?
3. What type of force holds the ions of dissolved ionic solid in aqueous solution?
What types of forces hold the molecules of a dissolved molecular solid in aqueous solution?
What type of force holds a nonpolar solute in a solution with a nonpolar solvent?
4. Ionic solids in which the ions are held very tightly in their crystal lattice likely have a relatively ______.
- low solvation energy
- high solvation energy
- low lattice energy
- high lattice energy
5. When the attractions between the water molecules and the solute particles of an aqueous solution are very strong, the solution likely has a relatively ________.
- low solvation energy
- high solvation energy
- low lattice energy
- high lattice energy