Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Spontaneity and Energy
Part c: The First Law of Thermodynamics
Part a:
What is Thermodynamics?
Part b:
Spontaneous Processes
Part c: The First Law of Thermodynamics
The Big Idea
The First Law of Thermodynamics states that energy is conserved — it can’t be created or destroyed, only moved or changed in form. In chemistry, this law links heat, work, and internal energy in every process.
Scientific Laws
If you have been exposed to much scientific thought and writing, you are likely familiar with the phrase “laws of nature.” Through years of experimentation and observation, scientists have discovered a variety of patterns of behavior in the natural world. From these “it-always-works-this-way” observations, scientific laws have emerged. A scientific law is a statement describing how nature behaves under specified conditions.
Here are a few scientific laws that you might be familiar with:
- An object at rest remains at rest unless acted upon by an unbalanced force.
- An unbalanced force acting upon an object causes an acceleration that is directly proportional to the strength of that force.
- The intensity of light observed at a given location is inversely proportional to the square of the distance from that location to the light source.
- When a chemical reaction occurs, the mass of all reactants is equal to the mass of all products.
- The volume of an ideal gas is directly proportional to the number of moles of the gas multiplied by the Kelvin temperature and inversely proportional to the pressure.
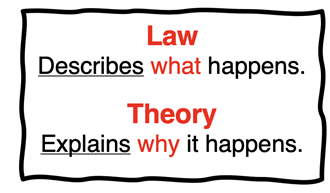
Scientific laws state how nature behaves. It’s a way of saying “This is how we have always observed nature to behave. You can expect it to behave this way next time.” Scientific laws do not explain why nature behaves the way it does. The explanation for why nature behaves as it does is associated with a theory. The last bullet point in our list above is a statement of
the ideal gas law from the
Gases Chapter of our
Chemistry Tutorial. It describes how an ideal gas behaves. The theory that explains why an ideal gas behaves as it does is the
Kinetic Molecular Theory (KMT). Like many theories, it provides a mechanistic view based on the motion of gas particles to explain why the ideal gas behavior is observed.
The First Law of Thermodynamics
There are four laws of thermodynamics. The so-called first law of thermodynamics can be stated as follows:
The total energy in the universe is constant.
Another common statement of the law is:
Energy cannot be created nor destroyed. It can only be converted from one form to another or transferred between a system and its surroundings.

Both statements are conveying the same idea. The idea is that total energy in the universe is conserved.
Conserved means to remain the same over the course of time. The total amount of energy doesn’t change and never will change. The form in which energy exists and its location may change, but the total amount of all its forms in all its locations is the same amount. Insofar as we have been able to make careful measurements and account for the total amount of energy in all its forms and locations, we have always observed conservation. And so, we are comfortable expressing these “it-always-works-this-way” observations as a law. The first law of thermodynamics is often referred to as the
law of conservation of energy.
Thinking in Terms of System and Surroundings
The first law of thermodynamics begs us to adopt a system and surroundings way of thinking. The
system is that part of the universe that is under study. In chemistry, that is most often the chemical reactants and products. We regard the system to be a
closed system; energy can transfer across the boundary of the system but matter or stuff remains closed within the system. The
surroundings includes all things outside the system of chemicals. Together the system plus the surroundings make up the universe. The total amount of energy in the universe is constant.
Changes take place within the system.
Bonds break. Bonds form. The energy of the system changes. For any energy changes occurring within the system, the opposite changes must occur outside the system in order for the total energy to be constant. If the system gains 100 kJ of energy, then the surroundings lose 100 kJ of energy. If the system loses 5000 kJ of energy, then the surroundings gain 5000 kJ of energy.
If an equation is needed to convey this idea, we could write:
∆Esystem = - ∆Esurroundings
The movement of energy across the boundary between system and surroundings is referred to as
energy transfer. When energy transfers into the system, there is a positive (+) change in the ∆E
system. And when energy transfers out of the system, there is a negative (-) change in the ∆E
system.
Energy Transfer: Heat and Work
Energy transfers across the boundary between the system and the surroundings can occur in the form of heat or in the form of work.
Heat is the transfer of energy from a high-temperature location to a low-temperature location. It is a temperature difference that drives the flow of heat between the two locations. Energy can be transferred into or out of a system when
work is done on the system by the surroundings or when
work is done on the surroundings by the system.
Heat Flow for Exothermic Reactions
Why does heat flow as a result of a chemical reaction? For an exothermic reaction, bonds break, atoms rearrange, and new bonds form to produce products that have a lower chemical potential energy than the reactants. This lowering of the chemical potential energy is balanced by the fact that the product particles will have a higher average kinetic energy than the reactants.
Kinetic energy and temperature are directly related; the temperature of the products is greater than the original temperature of the reactants and also greater than that of the surroundings. This difference in temperature causes heat to flow from the system to the surroundings. The system lowers its internal energy and the surroundings increase their temperature until both system and surroundings reach the same temperature.
Heat Flow for Endothermic Reactions
For an endothermic reaction, bonds break, atoms rearrange, and new bonds form to produce products that have a higher chemical potential energy than the reactants. This increase in the chemical potential energy balanced by the fact the product particles will have a lower average kinetic energy than the reactants.
Kinetic energy and temperature are directly related; the temperature of the products is less than the original temperature of the reactants and also less than that of the surroundings. This difference in temperature causes heat to flow from the surroundings to the system. The system increases its internal energy and the surroundings decrease their temperature until both system and surroundings reach the same temperature.
 Work Done On or By a System
Work Done On or By a System
Work is done by the surroundings on a system when the surroundings compress, squeeze, or otherwise exert a force on the system so that energy flows into it. Work done on a system transfers energy to the system and increases its internal energy. Suppose that the system is a collection of gases in a vertical cylinder that is sealed at its top end by a moveable piston. If a force is exerted downward upon the cylinder, then it will squeeze the gas, doing work upon the system. This would cause particles of the gas to move faster (more kinetic energy) or more chemical potential energy to be stored in the system.
Work can also be done on the surroundings by the system. This results in a transfer of energy out of the system into the surroundings. When a system of gases undergoes expansion, work is done by the system on the surroundings. The system loses energy and the surroundings gain energy.

First Law of Thermodynamics Equation
The first law of thermodynamics can be expressed in the form of the equation
∆E = Q + W
where
∆E is the change in energy of the system,
Q is the quantity of heat transferred to the system, and
W is the work done on the system by the surroundings.
The equation asserts that the energy change of a system is equal to the amount of heat transferred to it from the surroundings plus the amount of work done on it by the surroundings.

The equation is written such that the system gains energy when heat is transferred to it or work is done on it. Of course, it won’t always be the case that heat is transferred into the system; sometimes heat is transferred out of the system. For this reason, the value of Q can be either positive or negative. When heat is transferred out of the system Q is negative. Similarly, if work is done on the surroundings, W is negative.
Readers should be aware that there is another form of this equation that is written as
∆E = Q - W where
W is defined as the work done by the system on the surroundings.
Conserved Energy vs. Useful Energy
The first law of thermodynamics provides a confident assertion that the total amount of energy in the universe is conserved. This does not mean that the
usefulness of this energy is conserved. As energy is used, it is changed into less useful forms.
To illustrate the distinction between conservation of energy and energy usefulness, let’s consider the gasoline in your automobile. When your tank is full, you have several gallons of octane (C
8H
18) all in one location; it is a large concentration of chemical potential energy. As you use your car, you observe the fuel gauge decline from F to E. Once E is reached, there is no more chemical potential energy. It

has been transformed to heat and spread through the town, the state, the country, etc. Some heat exits your tailpipe with the exhaust gases and spreads about. Some heat warms your engine, only to later dissipate to the surroundings. Some heat warms the roads you travel along, only to later dissipate to the surroundings. Some heat warms the brake linings of your wheels, only to later dissipate to the surroundings. What was once a concentrated reservoir of very useful chemical potential energy is now an equivalent amount of thermal energy spread throughout the universe. The amount of energy is conserved. But the usefulness of the energy is not conserved.
There is no doubt that we can dig somewhere to get more fossil fuel (petroleum) to make more octane gasoline, should we choose to. But the reality is that we are using fossil fuels considerably faster than it is made. Eventually, the reserves we have will reach the same destination - dissipated thermal energy. We can (and should) put our best brains on the job of finding viable alternatives. But even so, none of this changes the fact that using energy changes it to less useful forms. Energy is conserved. Its usefulness isn’t. And that is a good segue to Lesson 2 on the topic of Spontaneity and Entropy.
 Photo Credit: National Archives, Project DOCUMERICA, Public Domain
Photo Credit: National Archives, Project DOCUMERICA, Public DomainBefore You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on First Law of Thermodynamics. Save it to a safe location and use it as a review tool.
Check Your Understanding of the First Law of Thermodynamics
Use the following questions to assess your understanding of what the first law of thermodynamics means. Tap the Check Answer buttons when ready.
1. For the following two statements, identify which is the law and which is the theory.
- Reaction rates increase with increasing reactant concentrations because collisions occur more frequently when reactant particles are more concentrated in the system.
- Reaction rates are observed to be higher when reactant concentrations are higher.
2. Identify all statements that are consistent with the first law of thermodynamics. Select all that apply.
- Systems always conserve their energy. The energy can change form but the total amount of all the forms is constant.
- For an exothermic process, the amount of energy lost by the system is equal to the amount of energy gained by the surroundings.
- The amount of energy in the system and the amount of energy in the surroundings is always equal to each other. Energy can transfer from the system to the surroundings but the amount in the system must equal the total amount in the surroundings.
- For an isolated system in which neither matter nor energy can enter or exit, the total energy of the system is a constant.
3. What are the two ways that energy is transferred between the system and the surroundings?
- As enthalpy and as heat.
- As kinetic energy and as potential energy.
- By heat flow and by work done.
- By car and by bus.
4. Which of the following statements are TRUE of exothermic reactions? Select all that apply.
- The temperature of the surroundings will ultimately increase.
- The internal energy of the system increases.
- The amount of internal energy lost by the system is equal to the amount of energy gained by the surroundings.
- The total energy of the system and surroundings before the reaction is greater than the total energy of the system and surroundings after the reaction.
5. Given our equation stating that
∆E = Q + W, describe the mechanism (heat, work, etc.) and the direction of energy transfer when ...
- Q is + and W is +
- Q is - and W is -
- Q is + and W is -
6. For the three scenarios described in Question #5, in which scenario can you be guaranteed that the system will increase its internal energy.
7. Use the word dissipate in a meaningful sentence.