Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 5: Hydrolysis of Salts
Part a: Predicting and Explaining the Acidity Level of Salts
Part a: Predicting and Explaining the Acidity Level of Salts
Part b:
Ka and Kb Values of Conjugate Acid-Base Pairs
The Big Idea
Dissolved salts don’t always form neutral solutions. Some form acidic or basic solutions through hydrolysis. The key is understanding how their ions interact with water. This lesson explains the principles and rationale and uses clear examples to help students master the hydrolysis of salts.
What Do Dissolved Salts Do?
 In Chemistry, a salt is simply an ionic compound. We readily associate NaCl with salts; but there are many other examples. Sodium acetate (NaC2H3O2), ammonium chloride (NH4Cl), calcium iodide (CaI2), potassium carbonate (K2CO3), and countless other substances are salts. A salt consists of a cation and an anion held together by ionic bonding.
In Chemistry, a salt is simply an ionic compound. We readily associate NaCl with salts; but there are many other examples. Sodium acetate (NaC2H3O2), ammonium chloride (NH4Cl), calcium iodide (CaI2), potassium carbonate (K2CO3), and countless other substances are salts. A salt consists of a cation and an anion held together by ionic bonding.
As we discussed in the Solutions Chapter of this Chemistry Tutorial, a soluble salt dissociates or splits apart to form solvated ions. For instance, sodium acetate (NaC2H3O2) dissociates as follows:
NaC2H3O2(s) → Na+(aq) + C2H3O2-(aq)
And ammonium chloride, NH4Cl, dissociates as follows:
NH4Cl(s) → NH4+(aq) + Cl-(aq)
The ions of a dissolved salt can do one of three things:
- The ion can donate a proton to water to form an acidic solution.
- The ion can accept a proton from water to form a basic solution.
- The ion can do nothing, in which case we could refer to it as a spectator ion.
In Lesson 5a, we want to inspect the interaction of these dissolved ions with water in order to predict whether the ions would form an acidic, basic, or neutral solution.
Hydrolysis of a Salt
In acid-base chemistry,
hydrolysis of a salt refers to the reaction of one or more its ions with water. The reactions involve proton transfer. The ammonium ion, NH
4+, is a relatively good proton donor. In aqueous solution, it wishes to donate an H
+ ion to a base. If there is no base present, then water would act as a base to receive the proton. The reaction is referred to as a hydrolysis reaction and described by the equation:
NH4+(aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
Since water becomes H
3O
+ when it receives the proton, we categorize the ammonium ion as having acidic properties. We would describe NH
4+ as being a weak acid.
The acetate ion, C
2H
3O
2-, is a relatively good proton acceptor. In aqueous solution, it wishes to accept an H
+ ion from an acid. If there is no acid present, then water would act as the acid to donate a proton to the acetate ion. The hydrolysis reaction is described by the equation:
C2H3O2-(aq) + H2O(l) ⇄ HC2H3O2(aq) + OH-(aq)
Since water becomes OH
- after donating a proton, we categorize the acetate ion as having basic properties. We would describe C
2H
3O
2- as being a weak base.
Hydrolysis of the dissolved ions of a salt explain why some salts are acidic and others are basic. The fact that some ions do not hydrolyze explains why some salt solutions are neutral.
What Ions are Neutral in Aqueous Solutions?

There are some ions which do not hydrolyze in solution. These include the anions of the strong, monoprotic acids.
The strong, monoprotic acids (discussed in Lesson 3a) are HCl, HBr, HI, HNO3, HClO4, and HClO3. The anions that are paired with H
+ in these acids do not hydrolyze in water. In fact, when we introduced strong acids, we wrote the dissociation equation with the irreversible reaction arrow:
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
The reverse reaction of the above dissociation does not occur because Cl
- is such a weak proton acceptor that it doesn’t even accept a proton from the very-willing-to-donate hydronium ion. In general, the anions of the strong, monoprotic acids are spectator ions in solution. They do not hydrolyze and do not contribute to the acidity or basicity of the solution.
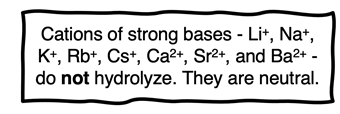
The cations of strong bases do not hydrolyze either.
The strong bases (discussed in Lesson 3a) are LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2. The cation paired with these OH
- ions do not accept or donate protons. They have neither acidic or basic qualities. In solution, they do not hydrolyze and act as spectator ions.
What Ions are Acidic in Aqueous Solutions?
In Lesson 3a, we used the Brønsted-Lowry model to write
chemical equations for the dissociation of weak bases in aqueous solutions. Weak bases are proton acceptors. They accept a proton (H
+ ion) from water and form their conjugate acid. Here are three weak base dissociation equations:
NH3(aq) + H2O(l) ⇄ NH4+(aq) + OH-(aq)
C5H5N(aq) + H2O(l) ⇄ C5H5NH+(aq) + OH-(aq)
CH3NH2(aq) + H2O(l) ⇄ CH3NH3+(aq) + OH-(aq)
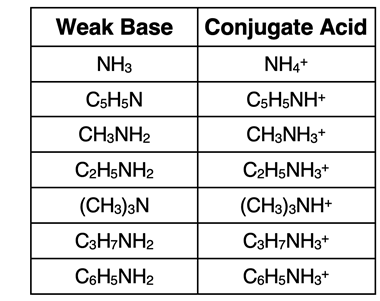
The
conjugate acid of the base is recognized as having a similar-looking formula with the exception that there is an extra H
+ in the
conjugate acid. The table at the right lists several weak bases and their
conjugate acids.
A
conjugate acid is an acid. And the conjugate acid of a weak base is a strong enough acid to interact with water and hydrolyze. Being an acid, it would donate a proton to water. Water, having received an H
+ ion, would become H
3O
+.
NH4+(aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
Since hydronium ions are produced by the hydrolysis of the weak acid, the pH is decreased. The conclusion can be made that any ion that is the conjugate acid of a weak base will have acidic properties and lower the pH of the solution.
What Ions are Basic in Aqueous Solutions?
We can apply the same Brønsted-Lowry reasoning to the
conjugate bases of weak, monoprotic acids. For instance, the acetate ion, C
2H
3O
2-, is the conjugate base of the weak acid, acetic acid (HC
2H
3O
2). It is formed by the acid’s dissociation in water:
HC2H3O2(aq) + H2O(l) ⇄ H3O+(aq) + C2H3O2-(aq)
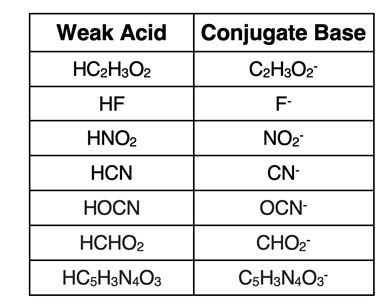
The
conjugate base of a weak acid is recognized as having a similar-looking formula with the exception that there is one less H
+ in the conjugate base. The table at the right lists several weak, monoprotic acids and their
conjugate bases.
A
conjugate base is a base. And the
conjugate base of a weak acid is a strong enough base to interact with water and hydrolyze. Being a base, it would accept a proton from water. Water, having donated an H
+ ion, would become OH
-.
C2H3O2-(aq) + H2O(l) ⇄ HC2H3O2(aq) + OH-(aq)
Since hydroxide ions are produced by the hydrolysis of the weak base, the pH is increased. The conclusion can be made that any ion that is the conjugate base of a weak, monoprotic acid will have basic properties and increase the pH of the solution.
How to Predict the Acidity Level of Salts
A salt consists of a cation and an anion. These ions will fall into one of
three categories:
- Spectator Ions (Neutral)
The anions of strong, monoprotic acids and the cations of strong bases fall into this category.
- Hydrolyze with H2O to form acidic solutions (Acidic)
The conjugate acids of weak bases fall into this category.
- Hydrolyze with H2O to form basic solutions (Basic)
The conjugate bases of weak, monoprotic acids fall into this category.
We can use the above conceptual understanding with the following
step-by-step procedure to predict the acidity level of salts:
- Determine the cation and the anion present in the salt.
- Determine if the cation falls into category A, B, or C above. That is, is it neutral, acidic, or basic.
- Determine if the anion falls into category A, B, or C above. That is, is it neutral, acidic, or basic.
- From your findings in Steps 2 and 3, categorize the salt as being acidic or basic. If the cation is acidic and the anion is basic (or vice versa), then additional reasoning must be done. This is discussed in the next section.
What if a Salt Contains an Acidic and a Basic Ion?
There will be occasions in which both the cation and the anion of a salt have opposing properties. For instance, the cation may be acidic and the anion may be basic. There are a few principles that can be considered to
break the tie in situations in which the cation and anion have opposing acid-base properties:
- The stronger that the weak acid is (i.e., the larger its Ka value), the weaker that its conjugate base will be.
- Conversely, the weaker that the weak acid is (i.e., the smaller its Ka value), the stronger that its conjugate base will be.
- The stronger that the weak base is (i.e., the larger its Kb value), the weaker that its conjugate acid will be.
- Conversely, the weaker that the weak base is (i.e., the smaller its Kb value), the stronger that its conjugate acid will be.
With these principles, comparing the strength of the weak acid (from which the basic anion is derived) to the strength of the weak base (from which the acidic cation is derived), you can reason towards whether the salt solution is acidic or basic.
Here are three situations to illustrate the reasoning.
Situation 1: Ammonium cyanide - NH4CN
- The cation is NH4+. It is the conjugate acid of the weak base NH3 (Kb = 1.8x10-5).
- The anion is CN-. It is the conjugate base of the weak acid HCN (Ka = 6.2x10-10).
- HCN is a weaker acid than NH3 is a base. Therefore, the strength of the conjugate base CN- is greater than the strength of the conjugate acid NH4+.
- Therefore, the greater strength of CN- as a base means that this salt solution will be slightly basic.
Situation 2: Ammonium chlorite - NH4ClO2
- The cation is NH4+. It is the conjugate acid of the weak base NH3 (Kb = 1.8x10-5).
- The anion is ClO2-. It is the conjugate base of the weak acid HClO2 (Ka = 1.1x10-2).
- HClO2 is a stronger acid than NH3 is a base. Therefore, the strength of the conjugate base ClO2- is less than the strength of the conjugate acid NH4+.
- Therefore, the greater strength of NH4+ as an acid means that this salt solution will be slightly acidic.
Situation 3: Ammonium acetate - NH4C2H3O2
- The cation is NH4+. It is the conjugate acid of the weak base NH3 (Kb = 1.8x10-5).
- The anion is C2H3O2-. It is the conjugate base of the weak acid HC2H3O2 (Ka = 1.8x10-5).
- HC2H3O2 is equally strong as an acid as NH3 is as a base. Therefore, the strength of the conjugate base C2H3O2- is equal to the strength of the conjugate acid NH4+.
- Because these two ions are of equal strength, the solution will be neutral.
Practice Questions with Answers - Predicting the Acidity Level of Salts
Determine if the aqueous solutions of the following salts are acidic, basic, or neutral. Think through your rationale for your conclusion. Then tap
Check Answer to see how you did. (If necessary, use the
listing of strong and weak acids and bases found in our Reference section.)
a. KBr
b. NaC
2H
3O
2
c. NH
4Cl
d. CaCl
2
e. CaF
2
f. C
2H
5NH
3Cl
g. C
5H
11NHF
Acid-Base Neutralization Revisited
In
Lesson 4b, we discussed pH curves for acid-base neutralization reactions. The equivalence point for such reactions was the point at which the acid-to-base mole ratio matched the acid-to-base coefficient ratio. The pH value at the equivalence point depended upon the products of the reaction. The products were described as “a salt and water.” Predicting the pH (or acidity level) at the equivalence point required an inspection of these products. Now that we have a better understanding of the hydrolysis of salts, let’s give a deeper explanation of the pH at these equivalence points.
Reaction Between a Strong Acid and a Strong Base
We learned that
the neutralization of a strong acid (like HCl) and strong base (like NaOH) results in an equivalence point with a pH of 7. Why? Since both acids and bases are strong, they exist as ions in solution. The H
+ ion is more appropriately described as the hydronium ion, H
3O
+. The complete ionic equation for the neutralization reaction is represented by the particle diagram below.
The products are a salt, NaCl, and water. The salt is composed of Na
+ and Cl
- ions. We have described Na
+ and Cl
- ions as neutral ions. In solution, they are spectator ions. And in this neutralization reaction, they are also spectator ions. Hydrolysis does not occur. Altogether, the products are pH-neutral, thus explaining the pH of 7 at the equivalence point for this strong acid-strong base neutralization.
Reaction Between a Weak Acid and a Strong Base
We learned that
the neutralization of a weak acid (like HF) and strong base (like NaOH) results in an equivalence point with a pH greater than 7. Why? Being a weak acid, the HF exists primarily as undissociated molecules. The NaOH exists as dissociated ions, Na
+ and OH
-. The complete ionic equation for the neutralization reaction is represented by the particle diagram below.
The products are a salt, NaF, and water. The salt is composed of Na
+ and F
- ions. The Na
+ ions are the spectator ions, consistent with being a cation of a strong base. The F
- is the conjugate base of the weak acid HF. As such, it is basic in solution. It would undergo hydrolysis, resulting in a pH greater than 7.0.
Reaction Between a Strong Acid and a Weak Base
We learned that
the neutralization of a strong acid (like HCl) and weak base (like NH3) results in an equivalence point with a pH less than 7. Why? Being a strong acid, the HCl exists in solution as hydronium ions (H
3O
+) and chloride ions (Cl
-). Being a weak base, the NH
3 primarily as undissociated molecules. The complete ionic equation for the neutralization reaction is represented by the particle diagram below.
The products are a salt, NH
4Cl, and water. The salt is composed of NH
4+ and Cl
- ions. The Cl
- ions are the spectator ions, consistent with being the anion of a strong acid. The NH
4+ is the conjugate acid of the weak base NH
3. As such, it is acidic in solution. It would undergo hydrolysis, resulting in a pH less than 7.0.
Using what we have learned on this page regarding the acidity levels of the ions of a dissolved salt, we can quickly analyze, explain, and predict the acidity level of a solution containing a salt.
What About Diprotic Acids?
In the discussion above, an emphasis has been placed upon the acids needing to be monoprotic. What’s the big deal about that?
The conjugate bases of a diprotic acid or a triprotic acid can act as either an acid or a base. We refer to such substances as being amphiprotic substances. They can behave as either a proton donor or a proton acceptor.
Consider carbonic acid - H
2CO
3 - a diprotic acid. It ionizes in a stepwise fashion. The conjugate base of its first ionization is the HCO
3- ion. HCO
3- is amphiprotic. It can donate a proton and become CO
32- or it can accept a proton and become H
2CO
3. There is one more trick that we need to learn in order to judge the most likely behavior of a salt containing the HCO
3- ion. We will learn that trick in
Lesson 5b.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Download our Study Card on Hydrolysis of Salts. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. In your own words, explain why some salts are neutral, others are acidic, and still others are basic. Think it through; then craft your explanation ... maybe even practice it on a friend (or enemy).
2. In Chemistry, a salt is ____.
- an option that you have for making food taste better.
- a physical or verbal or chemical attack launched against an adversary.
- a food additive that increases blood pressure.
- an ionic compound with a cation and an anion held together by ionic bonding.
3. Write the chemical equation for the hydrolysis of the following ions:
a. F
- ion:
b. OCl
- ion:
c. CH
3NH
3+ ion:
d. CO
32- ion:
4. Identify the conjugate base of the following
species:
a. HNO
2:
b. HOCN:
c. HIO
3:
d. HC
3H
5O
3:
5. Identify the conjugate acid of the following
species:
a. C
9H
7N:
b. C
6H
5NH
2:
c. HCO
3-:
6. Identify solutions of the following salts as being either acidic, basic, or neutral. Provide reasoning for your answer.
a. KC
2H
3O
2
b. NH
4Br
c. CH
3NH
3Br
d. Ca(CN)
2
e. RbCl