Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Acid and Base Dissociation
Part a: Strong vs. Weak Acids and Bases
Part a: Strong vs. Weak Acids and Bases
Part b:
Dissociation Constants
The Big Idea
Strong acids and bases are substances that completely dissociate into ions in water, while weak acids and bases only partially dissociate, creating an equilibrium between undissociated molecules and ions. This Chemistry Tutorial page presents the details regarding the differences between strong and weak acids and bases.
What is a Strong Acid?
We learned in Lesson 1b that acids are proton (H+ ion) donors. When dissolved in water, they undergo dissociation by transferring an H+ ion to water. For instance, hydrochloric acid, HCl, undergoes the following dissociation:
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
The products are the hydronium ion (H3O+) and the chloride ion (Cl-). The chloride ion is the conjugate base of the acid. The conjugate base is the substance formed when the acid loses its proton (H+ ion). In general, every acid having the generic formula HA will dissociate in water to form the hydronium ion and its conjugate base, A-.
HA(aq) + H2O(l) → H3O+(aq) + A-(aq)
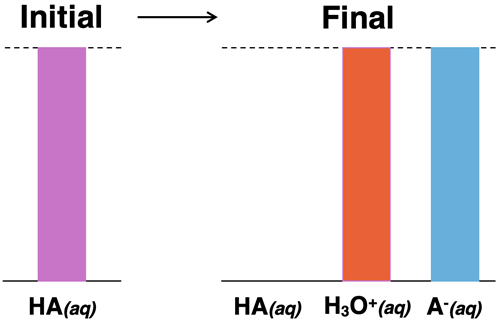
 An acid is often classified as being either a strong acid or a weak acid. A strong acid is an acid that completely dissociates into ions. Once dissociated, there are no remaining acid molecules present in the solution. All that is present are ions - H3O+ and A- ions. If 0.50 moles of HA are dissolved in water, then 0.50 moles of H3O+ ions and 0.50 moles of A- ions are produced.
An acid is often classified as being either a strong acid or a weak acid. A strong acid is an acid that completely dissociates into ions. Once dissociated, there are no remaining acid molecules present in the solution. All that is present are ions - H3O+ and A- ions. If 0.50 moles of HA are dissolved in water, then 0.50 moles of H3O+ ions and 0.50 moles of A- ions are produced.
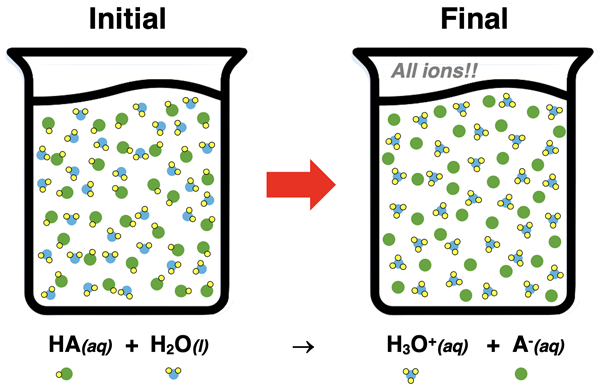
The conjugate base of a strong acid is a very weak base. For instance, HCl is a strong acid; it’s conjugate base, Cl-, is a very weak proton acceptor. The tendency of Cl- to accept a proton from another substance and turn back into HCl is very weak.
Dominant Forward Reaction: HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
Negligible Reverse Reaction: HCl(aq) + H2O(l) ← H3O+(aq) + Cl-(aq)
As such, for any strong acid, the forward reaction dominates the reverse reaction. The dissociation reaction can be described as going to completion. That is, the strong acid completely dissociates into ions.
 How are Weak Acids Different than Strong Acids?
How are Weak Acids Different than Strong Acids?
While strong acids completely dissociate in water, a weak acid only partially dissociates. The dissociation of a weak acid is a reversible reaction with an equilibrium position that lies far to the left. For a weak acid with the generic formula HA, the dissociation in water is represented by the equation:
HA(aq) + H2O(l) ⇄ H3O+(aq) + A-(aq)
This reaction does not go to completion as denoted by the reversible reaction arrow (⇄). At equilibrium, the solution consists primarily of the undissociated HA molecules. If 0.50 moles of HA are dissolved in water, then maybe 0.49 moles of HA molecules are present at equilibrium along with 0.01 moles of H3O+ ions and 0.01 moles of A- ions.
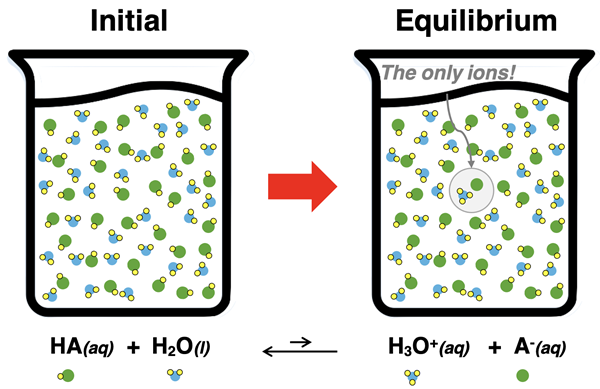
The conjugate base of a weak acid is a relatively strong base. For instance, HF is a weak acid; it’s conjugate base, F-, is a proton acceptor. Its proton-accepting abilities are greater than that of water. As such, the reverse reaction is more dominant than the forward reaction.
Weak Forward Reaction: HF(aq) + H2O(l) → H3O+(aq) + F-(aq)
Dominant Reverse Reaction: HF(aq) + H2O(l) ← H3O+(aq) + F-(aq)
Since F
- turning back into HF (the reverse reaction) dominates the equilibrium system, the dissociation is incomplete and only partial. Weak acids are acids that partially dissociate in water.
Examples of Strong and Weak Acids
There are seven common strong acids. They are hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), hydrobromic acid (HBr), hydroiodic acid (HI), perchloric acid (HClO4), and chloric acid (HClO3). Each of these will completely dissociate when dissolved in water. There are a countless number of weak acids. Some of the more common ones are listed in the table below. Weak acids are often described by an acid dissociation constant value (Ka). We will discuss Ka values in greater detail in Lesson 3b.
| Strong Acids |
Weak Acids |
| Hydrochloric acid (HCl) |
Acetic acid (HC2H3O2) |
| Sulfuric acid (H2SO4) |
Carbonic acid (H2SO3) |
| Nitric acid (HNO3) |
Phosphoric acid (H3PO4) |
| Hydrobromic acid (HBr) |
Nitrous acid (HNO2) |
| Hydroiodic acid (HI) |
Sulfurous acid (H2SO3) |
| Perchloric acid (HClO4) |
Hydrofluoric acid (HF) |
| Chloric acid (HClO3) |
Cyanic acid (HCN) |
Strong Bases
Like strong and weak acids, strong and weak bases are differentiated from one another in terms of the degree to which they dissociate when dissolved in water. Strong bases completely dissociate while weak bases only partially dissociate. When a strong base is dissolved in water, each molecules of the base will split into ions. There will be no undissociated particles of the base remaining. In contrast, a rather small percentage (often less than 1%) of the weak base will dissociate. The solution will mostly contain the undissociated base at equilibrium with only a few of the dissociated ions.
There are eight strong bases. Each is a hydroxide of an alkali metal or an alkaline earth metal. The strong bases are:
- Lithium hydroxide (LiOH)
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Rubidium hydroxide (RbOH)
- Cesium hydroxide (CsOH)
- Calcium hydroxide (Ca(OH)2)
- Strontium hydroxide (Sr(OH)2)
- Barium hydroxide (Ba(OH)2)
Each of these strong bases fits
the Arrhenius definition of a base; they contain hydroxide ions that are released into solution upon dissociation. Their dissociation equations are best written as follows:
NaOH(aq) → Na+(aq) + OH-(aq)
KOH(aq) → K+(aq) + OH-(aq)
Ca(OH)2(aq) → Ca2+(aq) + 2 OH-(aq)
Since strong bases completely dissociate, a solution prepared with a 0.500 mole NaOH in 1.00 L of solution will consist of 0.500 mole of Na
+ ions, 0.500 mole of OH
- ions, and 0.000 moles of undissociated NaOH.
Weak Base Dissociation
There are a variety of weak bases. The most common weak bases include ammonia (NH
3), methylamine (CH
3NH
2), ethylamine (C
2H
5NH
2), propylamine (C
3H
7NH
2), dimethylamine ((CH
3)
2NH), trimethylamine ((CH
3)
3N), pyridine (C
5H
5N), aniline (C
6H
5NH
2), and hydrazine (N
2H
4). Each of these Bronsted-Lowry bases are proton acceptors. When dissolved in aqueous solution, they accept a proton from a water molecule. The products are the
conjugate acid of the base and hydroxide ions (OH
-). Typical base dissociation equations look like these:
NH3(aq) + H2O(l) ⇄ NH4+(aq) + OH-(aq)
C5H5N(aq) + H2O(l) ⇄ C5H5NH+(aq) + OH-(aq)
CH3NH2(aq) + H2O(l) ⇄ CH3NH3+(aq) + OH-(aq)
In general, a generic base with the formula B reacts with water to form BH
+ and OH
- ions. The proton (H
+) is transferred from the water molecule to the base.
B(aq) + H2O(l) ⇄ BH+(aq) + OH-(aq)
Further information regarding base dissociation equations can be found in Lesson 1b.
All weak bases will only partially dissociate. Like weak acids, the reverse reaction is the dominant reaction in the reversible system, causing the equilibrium position to be on the reactant side. At equilibrium, the solution contains primarily undissociated base with minute amounts of the dissociated products.
Strong vs. Concentrated; Weak vs. Dilute

When we describe an acid or a base as being strong or weak, we are referring to its tendency to dissociate or ionize in water. These two terms should not be confused with the terms concentrated and dilute. A concentrated acid is
NOT a strong acid; and a dilute acid is
NOT a weak acid.
Concentrated and
dilute refer to how much acid or base is dissolved in the solution and is not related to its tendency to dissociate into ions. It is possible to have a dilute solution of a strong acid or of a strong base. This simply means that very little of the weak acid or base was dissolved in water. Similarly, it is possible to have a concentrated solution of a weak base. This means that a relatively large amount of weak base was dissolved in water. Concentrated and dilute refer to
the molarity of the substance in water. Strong and weak refer to the tendency of those substances to dissociate into ions.
Strong Acid and Base Calculations
![Six math equations relating pH, pOH, [H3O+], and [OH-].](/getmedia/51056cf5-b514-4927-9f13-4f363a1483af/Ch15L3a9.png?width=430&height=140)
Because strong acids and bases completely dissociate into ions, knowledge of the acid or base concentration is enough information to determine the concentrations of the ions. Because one of the ions will either be H
3O
+ (for acids) or OH
- (for bases), the pH of the solution can be predicted. Examples 1 through 4 demonstrate such predictions. The equations at the right will be helpful. If needed, review
Lesson 2b on
The pH Scale for help with
the relationships between [H3O+], [OH-], pH and pOH.
Example 1 - Calculating H3O+ Concentration of a Strong Acid
Determine the hydronium ion concentration for an aqueous solution of 0.41 M HNO
3(aq).
Solution:
HNO
3 is a strong acid. In water, it completely dissociates into hydronium and nitrate ions.
HNO3(aq) + H2O(l) → H3O+(aq) + NO3-(aq)
This means the concentrations of ions will be 0.41 M. Thus,
[H3O+] = 0.41 M.
Example 2 - Calculating the pH of a Strong Acid
A lab procedure calls for the use of a 0.25 M solution of HCl. Determine the ...
a. [OH
-]
b. [H
3O
+]
c. pH
d. pOH
Solution:
a. HCl is a strong acid that completely dissociates into ions.
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
The concentration of its ions are: [H
3O
+] = 0.25 M and [Cl
-] = 0.25 M. The part A answer is
[H3O+] = 0.25 M.
b. The hydronium ion and hydroxide ion are in solution and the product of their ion concentrations at 25°C (assumed) is always 1.0x10
-14 M
2.
[H3O+]•[OH-] = 1.0x10-14 M2
Because the [H
3O
+] is known (from part A), the [OH
-] can be calculated:
(0.25 M)•[OH-] = 1.0x10-14 M2
Divide each side by 0.25 M.
[OH-] = 1.0x10-14 M2 / 0.25 M
[OH-] = 4.0 x 10-14 M
c. The pH can be calculated using the equation pH = -log[H
3O
+].
pH = -log(0.25 M)
pH = 0.60
d. Since the pH is known (part C answer), the pOH is most easily calculated using the equation pH + pOH = 14.
0.60 + pOH = 14.00
Subtract 0.60 from each side of the equation.
pOH = 14 - 0.60
pOH = 13.40
Example 3 - Calculating the pH and pOH of a Strong Base
Mrs. Haber prepares a 0.40 M KOH
(aq) solution for an upcoming lab. Determine the ...
a. [OH
-]
b. [H
3O
+]
c. pH
d. pOH
Solution:
a. KOH is a strong base that complete dissociates into its ions:
KOH(aq) → K+(aq) + OH-(aq)
The concentration of the OH
- ions will be equal to the KOH molarity. Thus,
[OH-] = 0.40 M.
b. For any aqueous solution, there is both hydronium ions and hydroxide ions in solution and the product of their ion concentrations at 25°C (assumed) is always 1.0x10
-14 M
2.
[H3O+]•[OH-] = 1.0x10-14 M2
Because the [OH
-] is known (from part A), the [H
3O
+] can be calculated:
[H3O+]•(0.40 M) = 1.0x10-14 M2
Divide each side by 0.40 M.
[H3O+] = 1.0x10-14 M2 / 0.40 M
[H3O+] = 2.5 x 10-14 M
c. The pH can be calculated using the equation pH = -log[H
3O
+].
pH = -log(2.5x10-14 M)
pH = 13.60
d. Since the pH is known (part C answer), the pOH is most easily calculated using the equation pH + pOH = 14.
13.60 + pOH = 14.00
Subtract 13.60 from each side of the equation.
pOH = 14 - 13.60
pOH = 0.40
Example 4 - Calculating the pH and pOH of a Strong Base
A bottle at a lab station reads 0.025 M Ca(OH)
2. Perform calculations to determine the pH and pOH of this solution.
Solution:
Ca(OH)
2 is a strong acid that completely dissociates.
Ca(OH)2(aq) → Ca2+(aq) + 2 OH-(aq)
Consistent with the equation, 2 moles of hydroxide ions are produced for every mole of Ca(OH)
2 that dissociate. So, the concentration of OH
- is 0.050 M, twice that of the given concentration. The pOH can be calculated from the hydroxide ion concentration:
pOH = -log[OH-]
pOH = -log(0.050 M)
pOH = 1.30
The pH is most most easily calculated using the equation pH + pOH = 14.
pH + 1.30 = 14.00
Subtract 1.30 from each side of the equation.
pOH = 14 - 1.30
pOH = 12.70
These pH and pOH calculations can be difficult. Visit our
Tutorial page for detailed guidance with
the mathematical relationships between [H3O+], [OH-], pH and pOH.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding of Strong vs. Weak Acids and Bases
Use the following questions to assess your understanding of the distinction between strong and weak acids and bases. Tap the Check Answer buttons when ready.
1. Identify the following substances as being either a weak acid (WA), a strong acid (SA), a weak base (WB), or a strong base (SB).
a. KOH
b. HC
2H
3O
2
c. H
2SO
4
d. NH
3
e. HF
f. HNO
3
g. H
2C
2O
4
h. Ba(OH)
2
i. CH
3NH
2
2. Consider the following statements. Which statements describe strong acids and which statements describe weak acids? NOTE: some statements describe both strong and weak acids and will be included in each list.
- Has the general formula HA.
- Partially dissociates in water.
- Donates a proton to another substance.
- Dissociation reaction is mostly irreversible.
- Mostly dissociated ions are present in solution.
- The conjugate base of the acid is a relatively weak base.
- The conjugate base of the acid is a relatively strong base.
- The dissociation equilibrium position lies on the reactant side.
- A high concentration of undissociated acid molecules is present at equilibrium.
3. Suppose HX is a strong acid. A 1.0 M aqueous solution of HX will dissociate to produce a hydronium ion concentration that is ______ 1.0 M.
- much less than
- much greater than
- approximately equal to
4. Suppose HX is a weak acid. A 1.0 M aqueous solution of HX will dissociate to produce a hydronium ion concentration that is ______ 1.0 M.
- much less than
- much greater than
- approximately equal to
5. Trimethylamine, (CH
3)
3N, is a weak base. Its dissociation reaction would be:
- (CH3)3N(aq) ⇄ (CH3)3+(aq) + N-(aq)
- (CH3)3N(aq) + H2O(l) ⇄ (CH3)3NH+(aq) + OH-(aq)
- (CH3)3N(aq) + H2O(l) ⇄ (CH3)3NOH-(aq) + H3O+(aq)
- (CH3)3N(aq) + H2O(l) ⇄ (CH3)2NH+(aq) + CH3OH-(aq)
6. An aqueous solution of 2.0 M Ba(OH)
2 will have a hydroxide ion concentration of _____ M.
- Approximately 1.0 M
- Approximately 2.0 M
- Approximately 4.0 M
- ... nonsense! The [OH-] will be very, very small.
7. Determine the pH and the pOH of a 0.0052 M HNO
3 solution. Assume a temperature of 25°C.
8. Determine the pH and the pOH of a 1.5x10
-3 M KOH solution. Assume a temperature of 25°C.
9. Determine the hydroxide ion concentration for an aqueous solution made by dissolving 80.0 g of NaOH into 500.0 mL of water.