Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 4: Acid-Base Reactions
Part b: Neutralization Reactions Involving a Weak Acid or Base
Part a:
Reactions of a Strong Acid with a Strong Base
Part b: Neutralization Reactions Involving a Weak Acid or Base
Part c:
Titrations
The Big Idea
When a weak acid or a weak base are neutralized, the equivalence point does not have a pH of 7.0. Rather, the solution is basic or acidic due to the nature of the conjugate base or acid present as products. This lesson explains the phenomenon and then provides a short stoichiometry lesson pertaining to such neutralization reactions.
What Really Is Acid-Base Neutralization?
 We introduced the concept of acid-base neutralization in Lesson 4a, The strictest definition of an acid-base neutralization reaction requires that the acid and the base be of equal strength. For instance, the neutralization could include adding NaOH to a beaker containing a solution of HCl. Both the base and the acid are considered strong. If we could use a pH meter to measure the pH of the acid as the base is being added, we would observe increases in pH as more and more of the base is added. Eventually the moles of base would equal the moles of acid and the neutralization would be complete. The point at which neutralization is complete is known as the equivalence point. For a strong base neutralizing a strong acid, the pH of the contents of the beaker is 7.0 at the equivalence point. All that remains is a neutral salt and water.
We introduced the concept of acid-base neutralization in Lesson 4a, The strictest definition of an acid-base neutralization reaction requires that the acid and the base be of equal strength. For instance, the neutralization could include adding NaOH to a beaker containing a solution of HCl. Both the base and the acid are considered strong. If we could use a pH meter to measure the pH of the acid as the base is being added, we would observe increases in pH as more and more of the base is added. Eventually the moles of base would equal the moles of acid and the neutralization would be complete. The point at which neutralization is complete is known as the equivalence point. For a strong base neutralizing a strong acid, the pH of the contents of the beaker is 7.0 at the equivalence point. All that remains is a neutral salt and water.
The strictest definition of a neutralization reaction is not the only definition. A broader definition describes an acid-base neutralization reaction as being any reaction between an acid and a base that produces a salt and (sometimes) water. The acid and the base do not have to be of equal strength. By this broader definition, the reaction between a strong base and a weak acid is also considered a neutralization reaction. And so are the reaction between a strong acid and a weak base and the reaction between a weak acid and a weak base. Let’s explore these three other types of neutralization reactions. In the process, we will learn a new perspective on the term neutralization.
Reaction of a Strong Acid with a Weak Base - Proton Transfer Equations
Suppose that a solution of NH3 (weak base) is present in a beaker. Being a weak base, the contents primarily include undissociated NH3 molecules. And suppose HCl (a strong acid) is added to the base. The HCl is dissociated in water and exists as H3O+ ions and Cl- ions. What happens when H3O+(aq) ions, Cl-(aq) ions, and NH3(aq) molecules are in the same beaker? We think like Bronsted and Lowry and claim that the acid (H3O+) transfers a proton to the base (NH3) and a salt and water are formed. This is illustrated by the particle diagram.
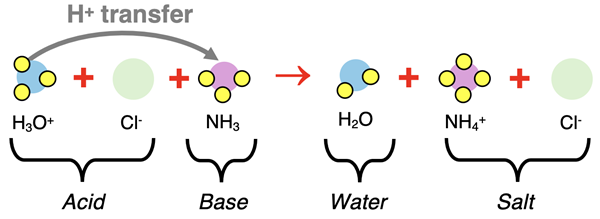
The above equation, in particle form, is the complete ionic equation. It shows what is happening in terms of the actual species that are present in solution. The chloride ion is a spectator ion and can be removed from the equation to form the net ionic equation.
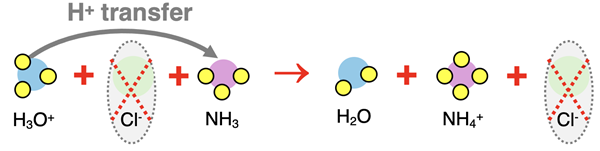
The three equations describing this acid-base neutralization reaction are:
Molecular Equation:
HCl(aq) + NH3(aq) → Cl-(aq) + NH4+(aq)
Complete Ionic Equation:
H3O+(aq) + Cl-(aq) + NH3(aq) → H2O(l) + NH4+(aq) + Cl-(aq)
Net Ionic Equation:
H3O+(aq) + NH3(aq) → H2O(l) + NH4+(aq)
 pH Curve for Strong Acid-Weak Base Reaction
pH Curve for Strong Acid-Weak Base Reaction
Let’s do a thought experiment. Suppose we set this reaction up with NH3 in a flask. And suppose we slowly add HCl (a strong acid) to the flask in an effort to neutralize the base. What will we observe if we equip the flask with a pH meter? How will the pH change and what will be the pH at the equivalence point? The graph at the right displays the answers.
Initially, the pH would be high since there is only acid in the flask. As HCl is added, the pH begins to lower as HCl reacts to reduce the amount of NH3 that is present. At a certain point during the process, a very noticeable change in pH occurs as the last of the NH3 is reacted away. Once the NH3 reacts away, the flask contains any additional HCl that was added beyond the equivalence point. This explains the very low pH near the end of the graph.
Amidst the sudden pH drop, there is a point where the number of moles of acid (H3O+ and Cl-) is equal to the number of moles of base (NH3). This is the equivalence point. For the addition of a strong acid to a weak base, the equivalence point occurs at a pH below 7. That is, when both reactants are used up and all that is in the flask are the products, the solution is slightly acidic. Why? Because the contents of the flask are neutral water and NH4+ ions. The NH4+ is the conjugate acid of NH3. As a conjugate acid, it is acidic. Unlike the reaction of a strong acid with a strong base, the reaction of a strong acid with a weak base has products that are not neutral; the products can affect the pH level of the solution.
Reaction of a Weak Acid with a Strong Base - Proton Transfer Equations
Now let’s consider the reaction of a weak acid with a strong base - hydrofluoric acid (HF) with sodium hydroxide (NaOH). The reaction produces a salt and water. The molecular equation is

Thinking in terms of particles, we will reason that the weak acid solution consists mostly of undissociated HF(aq) molecules. The strong base consists of dissociated ions - Na+(aq) and OH-(aq). So as the strong base is added to the weak acid, a reaction involving Na+(aq), OH-(aq), and HF(aq) would occur. As is always the case in an acid-base reaction, we can think in terms of proton transfer from the acid to the base. The HF molecule transfers a proton (H+ ion) molecule to the OH- ion and the conjugate acid and base are formed. The particle diagram represents this proton transfer.
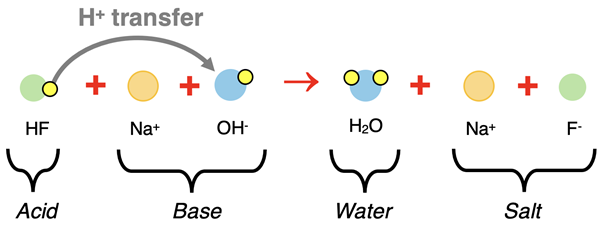
The above diagram is the particle representation of the complete ionic equation. The sodium ion appears on both reactant and product side. It is a spectator ion. By cancelling it from the complete ionic equation, the net ionic equation is produced
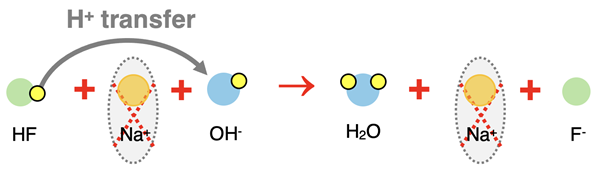
The three equations describing this acid-base neutralization reaction are:
Molecular Equation:
HF(aq) + NaOH(aq) → NaF(aq) + H2O(l)
Complete Ionic Equation:
HF(aq) + Na+(aq) + OH-(aq) → Na+(aq) + F-(aq) + H2O(l)
Net Ionic Equation:
HF(aq) + OH-(aq) → F-(aq) + H2O(l)
 pH Curve for Weak Acid-Strong Base Reaction
pH Curve for Weak Acid-Strong Base Reaction
Let’s repeat our thought experiment for the weak acid (HF) in a flask, to which we will add the strong base, NaOH. Once more, we will imagine a pH meter in the flask that monitors the pH as the base is added to it. What will be observed?
Initially, the pH will be low since there is only acid in the flask. As the NaOH is added, the HF will react with it, thus reducing the amount of HF and slowly increasing the pH. As more moles of NaOH are added, the equivalence point is approached. Suddenly there is a steep rise in the pH. At the equivalence point, when the number of moles of weak acid is equal to the number of moles of strong base, the pH is greater than 7.0. Why? Because at this point, all that is in the flask is neutral water and F- ions. The F- is the conjugate base of the weak acid, HF. As a conjugate base, it is basic and causes the pH to be higher than 7.0.
Why Neutralization isn’t Always Neutral?
A novice intuition usually wants a neutralization reaction to result in a neutral solution - that is, a pH of 7.0. But as we’ve seen above, this only occurs when equal strength acids and bases, like HCl and NaOH, react.
The word neutralization has come to be used in a broader sense. Neutralization doesn’t have to mean that the final solution (at the equivalence point) ends up as pH-neutral. Neutralization can also mean that the acid’s ability to donate protons and base’s ability to accept protons have both been used up. The acid that was originally present has been reacted away by the base that was added to it (or vice versa). At the equivalence point, that original acid and original base are no longer present. They have been used up. They have reacted away. They have been neutralized. The pH level at the equivalence point is then determined by the products of the reaction. And those products can be acids or bases and thus affect the pH at the equivalence point.
| Acid-Base Neutralization Reaction |
| Reactants |
At Equivalence Point |
| Acid |
Base |
pH |
Dominant Species in Solution |
Strong
e.g., HCl
|
Strong
e.g., NaOH
|
7.0
|
A neutral salt & H2O
|
Strong
e.g., HCl
|
Weak
e.g., NH3
|
< 7.0
|
Conjugate acid of a weak base & H2O
|
Weak
e.g., HF |
Strong
e.g., NaOH |
> 7.0 |
Conjugate base of a weak acid & H2O |
So, while the resulting solution isn’t always pH-neutral, the acidic and basic properties of the original reactants are gone. That’s why chemists are comfortable with the broader definition of an acid-base neutralization reaction. Acid-base neutralization involves balancing the moles of acid with the moles of base such that neither of the initial reactants are remaining. As for the pH-result, that simply depends on what the products are of the reaction.
Reaction of a Weak Acid with a Weak Base - Proton Transfer Equations
The last scenario to be discussed is the reaction between a weak acid and a weak base. Like all previous reactions, there is a proton transferred from the acid to the base to form the conjugate base and the conjugate acid. Because both the acid and the base are weak, solutions of these will contain undissociated molecules.
Let’s consider the reaction between the weak acid HF and the weak base NH3. A proton (H+ ion) is transferred from the HF to the NH3.
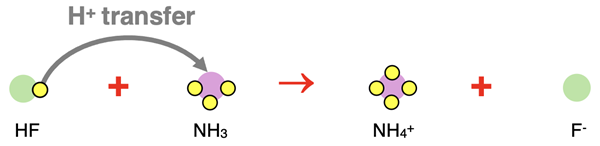
The products are F-, the conjugate base of HF, and NH4+, the conjugate acid of NH3. The molecular equation, complete ionic equation, and net ionic equation all look the same.
HF(aq) + NH3(aq) → F-(aq) + NH4+(aq)
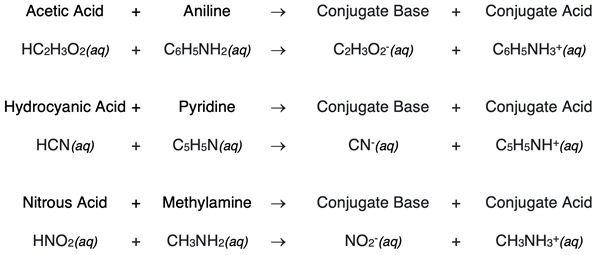
Other weak acid - weak base neutralization reactions will show the same pattern of a proton transfer from the acid to the base to form the conjugate base and conjugate acid. Here are some more examples:
Reaction of a Weak Acid with a Weak Base - pH at the Equivalence Point
What is the pH of the solution when the moles of weak acid is equal to the moles of weak base? Predicting the pH at the equivalence point for a weak acid-weak base reaction is done like any other neutralization reaction - inspect the products to see what is present. But the prediction is complicated by the fact that there is both an acid and a base present at the equivalence point. Predicting the pH requires a comparison of the acid’s strength to the base’s strength. And that means we need to inspect the Ka and Kb values of the products.
 In the case of HF reacting with NH3, the products are F- ion and NH4+ ion. Their dissociation constants are:
In the case of HF reacting with NH3, the products are F- ion and NH4+ ion. Their dissociation constants are:
F-: Kb = 1.5 x 10-11
NH4+: Ka = 5.6 x 10-10
The K
a value of the conjugate acid is slightly larger than the K
b value of the conjugate base. Thus, the equivalence point occurs at a pH value that is slightly less than 7.0 - on the acidic side of neutral.
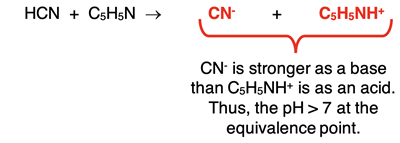
In the case of HCN reacting with C
5H
5N, the products are F
- ion and NH
4+ ion. Their dissociation constants are:
CN-: Kb = 1.6 x 10-5
C5H5NH+: Ka = 6.7 x 10-6
The K
b value of the conjugate base is slightly larger than the K
a value of the conjugate acid. Thus, the equivalence point occurs at a pH value that is slightly greater than 7.0 - on the basic side of neutral.
Psst. Want to Know a Secret?
You might be wondering
where did these Ka and Kb values for the conjugate acid and conjugate base come from? Curious minds deserve to know. It ends up that the product of the K
a and K
b values for any conjugate acid-base pair is equal to 1.0 x10
-14 (the K
w of water).
For conjugate acid-base pairs: Ka • Kb = 1.0 x10-14
The K
a of HF is known to be 6.6x10
-4 (see our
Reference page). The K
b of F
- is (1.0 x10
-14)/(6.6x10
-4).
The K
b of NH
3 is known to be 1.8x10
-5 (see our
Reference page). The K
a of NH
4+ is (1.0 x10
-14)/( 1.8x10
-5).
Now you know the secret!
Neutralization Stoichiometry Revisited
For neutralization to occur, the ratio of the number of moles of acid to the number of moles of base must equal the ratio of coefficients in the balanced chemical equation for the neutralization reaction. This ratio is often 1:1 as in the case for HF and NH3.
HF(aq) + NH3(aq) → F-(aq) + NH4+(aq)
But in cases in which the acid is diprotic (e.g., H2C2O4) or triprotic (e.g., H3PO4), and in cases in which the base contains two hydroxide ions (e.g., Ca(OH)2, one must be careful to account for possible 2:1, 3:1, or 1:2 ratios. For instance, if oxalic acid, H2C2O4, reacts with NaOH, the balanced chemical equation is:
H2C2O4(aq) + 2 NaOH(aq) → Na2C2O4(aq) + 2 H2O(l)
To neutralize 1 mole of H2C2O4, 2 moles of NaOH must be reacted with it. The mole ratio of acid to base is 1:2.
Similarly, the reaction of acetic acid, HC2H3O2, with calcium hydroxide, Ca(OH)2, has a 2:1 mole ratio of acid to base.
2 HC2H3O2(aq) + Ca(OH)2(aq) → Ca(C2H3O2)2(aq) + 2 H2O(l)
The stoichiometry of acid-base neutralization reactions is like any stoichiometry problem – balanced chemical equations and mole ratios (coefficients) are essential to determining how much acid and base will react with each other.
Suggested Review: Solution Stoichiometry || Strong Acid-Strong Base Stoichiometry
Example 1 - Calculating the Volume of Base
A beaker contains 250.0 mL of 0.400 M H2C2O4. What volume of 0.250 M NaOH must be added to it for complete neutralization?
Solution:
We will approach the answer by using the factor label method. The coefficients allow us to relate moles of acid to moles base. There is a 1:2 acid-to-base mole ratio.
H2C2O4(aq) + 2 NaOH(aq) → Na2C2O4(aq) + 2 H2O(l)
The molarities allow us to relate volumes (in L) to moles. The conversion factors are first set up to cancel units while converting from the volume of the H2C2O4 solution to the volume of the NaOH solution. As is our practice, we leave numbers out of the conversion factors. The factor label method is all about unit cancellation. We focus on units first and numbers later.
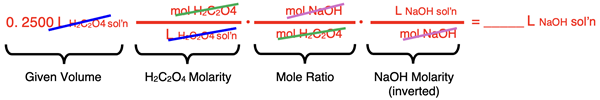
Once the conversion factors are arranged appropriately, numbers can be placed in the numerators and denominators. Then (and not until then) the calculator can be used to determine the answer.

The answer is 0.800 L or 800. mL of NaOH solution must be added to neutralize the oxalic acid.
Example 2 - Calculating the Molarity of Acid
An acetic acid (HC2H3O2) solution of unknown concentration is neutralized by the addition of 45.8 mL of 0.100 M Ca(OH)2. If the volume of the acid solution is 50.0 mL, determine its concentration.
Solution
The factor label method will be used to determine the moles of HC2H3O2 in the solution. Then the moles and the given volume can be used to calculate the molarity. The acid-to-base mole ratio is 2:1 as shown by the balanced equation.
2 HC2H3O2(aq) + Ca(OH)2(aq) → Ca(C2H3O2)2(aq) + 2 H2O(l)
The conversion factors are first set up to convert from the volume of Ca(OH)2 to the moles of HC2H3O2.
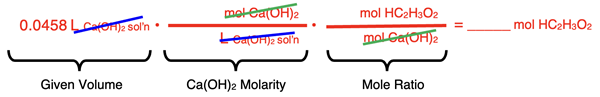
Numbers are placed into the numerators and denominators and the moles of HC2H3O2 are determined.
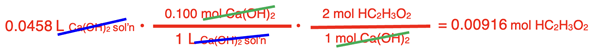
Now the molarity of HC2H3O2 can be calculated by dividing the moles by the given volume (in L) of the acid solution.
Molarity = moles HC2H3O2 / Volume of Solution
Molarity = (0.00916 mol HC2H3O2) / (0.0500 L)
Molarity = 0.0183 M
Next Up
A common application of acid-base stoichiometry is the analytic procedure known as volumetric analysis. Most students will know this as titration. On the next page of Lesson 4, we will discuss Titration in a Chemistry Lab. Let’s meet up there after you take some time to try the Practice and Reinforcement ideas below.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding of Acid-Base Neutralization Reactions
Use the following questions to assess your understanding of how the strength of the acid or base affects the pH at the equivalence point. Tap the Check Answer buttons when ready.
1. Complete the following paragraph:
When the weak acid HClO2 is reacted with the strong base NaOH, a proton is transferred from the _______ (HClO2, OH-) to the _______ (HClO2, OH-). As a result, the HClO2 becomes ___________ (H2ClO2+, ClO2-, H2O, H3O+) which is its conjugate _________ (acid, base, salt). As a result of the proton transfer, the OH- becomes ___________ (H2ClO2+, ClO2-, H2O, OH2-) which is its conjugate _________ (acid, base, salt). At the equivalence point of the reaction, the pH will be _________ (greater than, less than, equal to) 7.0.
2. Complete the following paragraph:
When the strong acid HCl is reacted with the weak base C
5H
5N, a proton is transferred from the _______ (H
3O
+, C
5H
5N) to the _______ (H
3O
+, C
5H
5N). As a result, the H
3O
+ becomes ___________ (H
2O, OH
-, H
4O
2+, H
2O
2) which is its conjugate _________ (acid, base, salt). As a result of the proton transfer, the C
5H
5N becomes ___________ (C
5N
5+, C
5H
4N
-, C
5H
5NH
+, H
2O) which is its conjugate _________ (acid, base, salt). At the equivalence point of the reaction, the pH will be _________ (greater than, less than, equal to) 7.0.
3. Complete the following table for a weak acid-weak base neutralization reaction. The first row is done as an example. (NOTE: the weaker than a weak acid is, the stronger its conjugate base will be. And the weaker that a weak base is, the stronger that its conjugate acid will be.)
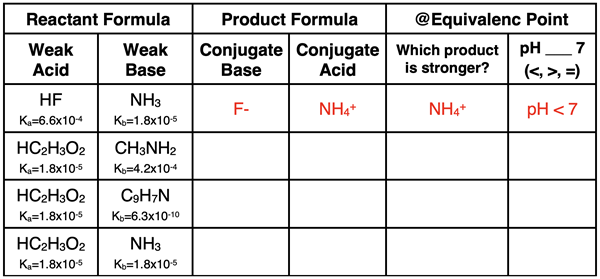
4. Complete the following statements by entering a numerical value in the blank.
- To neutralize 0.030 mol HC2H3O2, ________ mol of KOH must be added to it.
- To neutralize 0.030 mol HC2H3O2, ________ mol of Ba(OH)2 must be added to it.
- To neutralize 0.030 mol H2C2O4, ________ mol of KOH must be added to it.
- To neutralize 0.030 mol H2C2O4, ________ mol of Ba(OH)2 must be added to it.
- To neutralize 0.030 mol H3PO4, ________ mol of LiOH must be added to it.
- To neutralize 0.030 mol H3PO4, ________ mol of Ba(OH)2 must be added to it.
5. A Ca(OH)
2 solution is made by dissolving 7.41 g Ca(OH)
2 in 0.500 L of water. The molar mass of Ca(OH)
2 is 74.1 g/mol.
- Determine the moles of HC2H3O2 that must be added to the solution for complete neutralization.
- Determine the moles of H2C2O4 that must be added to the solution for complete neutralization.
- Determine the volume (in L) of 0.100 M HC2H3O2 that must be added to the solution for complete neutralization.
- Determine the volume (in L) of 0.200 M HC2H3O2 that must be added to the solution for complete neutralization.
- Determine the volume (in L) of 0.400 M HC2H3O2 that must be added to the solution for complete neutralization.
- Determine the volume (in L) of 0.400 M H2C2O4 that must be added to the solution for complete neutralization.