Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 4: Acid-Base Neutralization Reactions
Part a: Reactions of a Strong Acid and a Strong Base
Part a: Reactions of a Strong Acid with a Strong Base
Part b:
Neutralization Reactions Involving a Weak Acid or Base
Part c:
Titrations
The Big Idea
Learn what acid-base neutralization reactions are, how to write molecular and net ionic equations, and how to perform neutralization stoichiometry using moles and using volumes and molarities.
What is an Acid-Base Neutralization Reaction?
In Lesson 3 of our Acids and Bases Chapter, we discussed the dissociation of acids and bases in water. We wrote dissociation equations that expressed the nature of an acid as a proton (H+ ion) donor and the nature of a base as a proton (H+ ion) acceptor.
Acid Dissociation: HF(aq) + H2O(l) ⇄ H3O+(aq) + F-(aq)
Base Dissociation: NH3(aq) + H2O(l) ⇄ NH4+(aq) + OH-(aq)
Each dissociation reaction demonstrated one of two tendencies:
- the acid donated a proton to water, which acted as a base or proton acceptor.
- water, which acted as an acid or proton donor, donated a proton to the base.
Water is unique in that it is
amphiprotic; it can act as either an acid or a base. In Lesson 4a, we will explore reactions that do not depend on water’s
amphiprotic nature. We will observe strong acids reacting with strong bases. As we explore these new reactions, we will continue to think in terms of
proton transfer.

We will begin with what is commonly called an acid-base neutralization reaction.
Strictly speaking, an
acid-base neutralization reaction is a reaction between an acid and a base (of equal strength) to form a salt and water. For instance, the reaction between a strong acid like HCl and a strong base like NaOH is an example of an acid-base neutralization reaction. The chemical equation for the reaction is sometimes written as:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Acid + Base → Salt + Water

In this reaction, the acid is HCl, the base is NaOH, and the
salt is NaCl. Both the dissolved NaCl and the H
2O are neutral. Once all the acid and base has reacted, the final solution (the products) are neutral with a pH of 7.0. For our
strict definition, this is the reason we include the word neutralization to describe such reactions. (As we will see on the next page of this Lesson, there are broader definitions that do not restrict the reactants to being strong acids and strong bases.)
We first introduced the concept of a
salt in the
Solutions Chapter of this
Chemistry Tutorial. The salt in the above reaction is NaCl, which most of us associate with the crystals we shake onto our French fries. But more generally, the salt referred to in this discussion is any ionic compound consisting of a cation and an anion. So, the following reaction between HNO
3 (a strong acid) and KOH (a strong base) meets the strictest definition of an acid-base neutralization reaction. The salt is KNO
3.
HNO
3(aq) + KOH
(aq) → KNO
3(aq) + H
2O
(l)
Writing Molecular, Complete Ionic, and Net Ionic Equations
The above chemical equations are referred to as
molecular equations. The reactants are written in molecular form even though we know that the
species present in an aqueous solution of a strong acid and a strong base are ions and not molecules. The distinction between molecular equations, complete ionic equations, and net ionic equations was first introduced in Chapter 8 of our
Chemistry Tutorial. (Review
Three Equations for One Event.) Complete and net ionic equations represent the reactants and products as ions ... if that’s the most appropriate representation of how they exist in solution.
Let’s begin with the reaction between HCl
(aq) and NaOH
(aq). Because HCl completely dissociates when dissolved in water
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
it exists in solution as H
3O
+(aq) and Cl
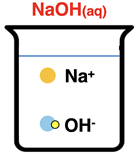
-(aq) ions. Sodium hydroxide (NaOH), like all the strong bases, is a metal hydroxide, a solid at 25°C, and an Arrhenius base. When dissolved in water, it dissociates into ions.
NaOH(s) → Na+(aq) + OH-(aq)
An aqueous solution of NaOH consists of Na
+(aq) and OH
-(aq) ions. We can think of the
species involved in the reaction as H
3O
+(aq), Cl
-(aq), Na
+(aq), and OH
-(aq) ions.

So what will these four ions do? How will they react? We answer this question by thinking in the same way we have thought the entire chapter. We ask two questions:
- Which of the four ions has a proton (H+ ion) to donate? That is, which is the acid?
- Which of the four ions would like to accept a proton (H+ ion)? That is, which is the base?
The answer is that H
3O
+, which has been associated with acidity the entire chapter, is the acid. It wishes to donate a proton. And OH
-, has been associated with basicity, is the base; it wishes to accept a proton. So now we can write the complete ionic equation, showing all ions and showing the acid (H
3O
+) donating a proton (H
+) to the base (OH
-).
Complete Ionic Equation:
H3O+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → H2O(l) + Cl-(aq) + Na+(aq) + H2O(l)
If we inspect the complete ionic equation for this
proton-transfer event, we observe two ions that are not changing - Na
+(aq) and Cl
-(aq). They are present on both the reactant and the product side of the complete ionic equation. These are referred to as
spectator ions. As in a sporting event, spectators are not involved in the action. If we eliminate them from the complete ionic equation, we generate a net ionic equation.
Net Ionic Equation:
H
3O
+(aq) +
Cl-(aq) +
Na+(aq) + OH
-(aq) → H
2O
(l) +
Cl-(aq) +
Na+(aq) + H
2O
(l)

or
H
3O
+(aq) + OH
-(aq) → 2 H
2O
(l)
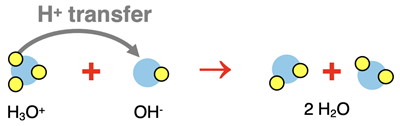
Here is a summary of the three equation types for the neutralization reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH):
Molecular Equation:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Complete Ionic Equation:
H3O+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → H2O(l) + Cl-(aq) + Na+(aq) + H2O(l)
Net Ionic Equation:
H3O+(aq) + OH-(aq) → 2 H2O(l)
A close inspection of the two ionic equations reveals the main point of our analysis: acid-base neutralization reactions involve
the transfer of a proton (H+ ion).
|
A Shortcut That Works
There is a shortcut that is often used for this topic. HCl is represented as H+ ions and Cl- ions rather than as H3O+ and Cl- ions. The net ionic equation becomes
H+(aq) + OH-(aq) → H2O(l)
The shortcut works and may even make more intuitive sense than our longcut. However, it is important to remember that there are no free protons (H+ ions) floating around in solution. They are always attached to a water molecule and exist as H3O+ ions. The longcut also helps to reinforce the Brønsted–Lowry model of acid-base reactions as proton transfer events that result in the formation of the conjugate acid and the conjugate base. |
Writing Acid-Base Neutralization Equations (Strong Acid + Strong Base)
A likely task encountered in most Chemistry courses is the task of analyzing the stoichiometry of an acid-base neutralization reaction. Like any stoichiometry problem, the analysis begins with a balanced chemical equation. A molecular equation is usually the most feasible equation for such analyses. It can be written by remembering that an acid-base neutralization reaction involves an acid reacting with a base to produce a salt and water.

Writing such an equation requires the following steps:
- Identify the formula of the acid and the formula of the base.
- Write these formulae as the two reactants.
- Write the formula of the salt. It is formed by the cation of the base and the anion of the acid. Be sure to include the proper subscripts. Review Formula Writing if necessary.
- Write the products as the salt plus water (H2O). The skeleton equation is now complete.
- Use coefficients to balance the equation.
Example 1 - Writing Balanced Chemical Equations
For the reactions described below, write the balanced molecular equation. Then tap on Check Answer to check your answer.
Write a balanced chemical equation for the acid-base neutralization reaction between ...
- HNO3 and KOH.
- H2SO4 and NaOH.
- HCl and Ba(OH)2.
- HNO3 and Ca(OH)2.
- H2SO4 and Ca(OH)2.
Neutralization Stoichiometry - Moles
Suppose that a lab student starts with a flask containing 0.50 mol of HCl(aq). Gradually, the student adds NaOH(aq) to the flask. An acid-base neutralization reaction begins to occur. How much base (NaOH) must be added to completely neutralize the acid (HCl) in the flask? Most students quickly grasp the answer - 0.50 mol of NaOH(aq) must be added to neutralize 0.50 mol of HCl(aq). It’s straightforward stoichiometry based on the balanced chemical equation:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
 The equation calls for a 1:1 mole ratio of the acid to the base. Once all the HCl is reacted away by an equal number of moles of NaOH, all that is left in the flask is two neutral substances - NaCl and H2O. Neutralization has occurred.
The equation calls for a 1:1 mole ratio of the acid to the base. Once all the HCl is reacted away by an equal number of moles of NaOH, all that is left in the flask is two neutral substances - NaCl and H2O. Neutralization has occurred.
The point at which neutralization occurs is called the equivalence point. For the above reaction, neutralization occurs when the mole ratio is 1:1.
Now let’s consider a different neutralization reaction:
H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 H2O(l)
Notice that the mole ratio is 1:2. Because the H2SO4 has two ionizable protons, twice as many moles of NaOH are required to neutralize it. If a flask contains 0.50 mol of H2SO4(aq), then 1.00 mol of NaOH would be required to neutralize it. Once again, it is straightforward stoichiometry based on the balanced chemical equation.
 In the case of H2SO4 reacting with NaOH, the equivalence point occurs when the moles of NaOH is twice the moles of H2SO4. That’s not exactly equal. Why call it the equivalence point? Because at the equivalence point, the moles of H3O+ ions (or H+ ions if we use the shortcut) equals the moles of OH- ions. Equivalence point always makes more sense when you imagine the neutralization as involving the ions.
In the case of H2SO4 reacting with NaOH, the equivalence point occurs when the moles of NaOH is twice the moles of H2SO4. That’s not exactly equal. Why call it the equivalence point? Because at the equivalence point, the moles of H3O+ ions (or H+ ions if we use the shortcut) equals the moles of OH- ions. Equivalence point always makes more sense when you imagine the neutralization as involving the ions.
Here's more examples of neutralization stoichiometry. As you read through, think in terms of ions - H3O+ (or H+) and OH- ions.
- To neutralize 0.020 mol HNO3, 0.020 mol of KOH must be added to it.
- To neutralize 0.020 mol H2SO4, 0.040 mol of KOH must be added to it.
- To neutralize 0.020 mol HCl, 0.020 mol of KOH must be added to it.
- To neutralize 0.020 mol HCl, 0.010 mol of Ca(OH)2 must be added to it.
- To neutralize 0.020 mol H2SO4, 0.020 mol of Ca(OH)2 must be added to it.
Example 2 - Determining the Moles of Base
How many moles of NaOH must be added to a flask to neutralize 0.25 moles of H2SO4?
Solution:
The balanced chemical equation is:
H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 H2O(l)
From the coefficients, 2 moles of NaOH are needed for every 1 mole of H2SO4. So, 0.50 moles of NaOH must be added to neutralize the 0.25 moles of H2SO4.
Example 3 - Determining the Moles of Acid
A flask contains 0.0020 moles of Ca(OH)2. How many moles of HCl must be added to neutralize the base.
Solution:
The balanced chemical equation is:
2 HCl(aq) + Ca(OH)2(aq) → CaCl2(aq) + 2 H2O(l)
From the coefficients, 2 moles of HCl are needed for every 1 mole of Ca(OH)2. So, 0.0040 moles of HCl must be added to neutralize the 0.0020 moles of Ca(OH)2.
Example 4 - Determining the Moles of Acid
A beaker contains 4.0 grams of NaOH (molar mass = 40.0 g/mol) dissolved in 0.500 L of water. How many moles of HCl must be added to the beaker to neutralize the NaOH.
Solution:
The balanced chemical equation is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Based on the mass and the molar mass of NaOH, there is 0.10 mol of NaOH in the beaker. From the coefficients, 1 mole of HCl are needed for every 1 mole of NaOH. So, 0.10 moles of HCl must be added to neutralize the 4.0 g or 0.10 moles of NaOH.
Neutralization Stoichiometry - Volume and Molarity
When chemists do mathematics with aqueous solutions, they usually think in terms of volume and molarity. Molarity is the concentration of solute, expressed in moles of solute per liter of solution. The unit is mol/L and is abbreviated as M. A 2.5 M solution of HCl contains 2.5 mol of HCl per every 1.0 L of solution. By multiplying the volume of the solution by the molarity, the number of moles of solute can be determined.
# moles of Solute = Volume in L • Molarity
A 0.10-L solution of 2.5 M HCl contains 0.25 mol of HCl.
# mol HCl = (0.10 L sol’n) • (2.5 mol HCl / 1 L sol’n) = 0.25 mol HCl
Many analyses of acid-base-neutralization reactions involve the use of volume and molarity. If needed, review the following Lessons from the Solutions Chapter:
Molarity | Solution Stoichiometry
Example 5 - Determining the Volume of Acid
A flask contains 250 mL of 0.10 M NaOH. What volume of 0.500 M HCl must be added to the flask to neutralize it?
Solution Approach #1
We will show two different approaches to this problem. Both rely on an understanding that the moles of HCl must equal the moles of NaOH at the equivalence point.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
The moles of NaOH can be determine from the volume (0.250 L) and the molarity (0.10 M):
# moles NaOH = (0.250 L)•(0.10 mol NaOH / 1 L) = 0.025 mol NaOH
To neutralize this NaOH, 0.025 mol of HCl must be added to the flask. Using the molarity of HCl as a conversion factor, the volume can be determined:

To neutralize the NaOH, 0.050 L (50. mL) of the HCl solution must be added to the flask.
Solution Approach #2
The second approach to the problem relies on the factor label method. The approach is like that of any stoichiometry problem and relies on molarities and mole ratios as conversion factors. As we have always recommended, begin the process by arranging the conversion factors with a focus on unit cancellation. Avoid the use of numbers; the goal is to set up factors to convert from the given 250 mL (0.250 L) of the NaOH solution to the volume of the HCl solution. The conversion factor set up is:
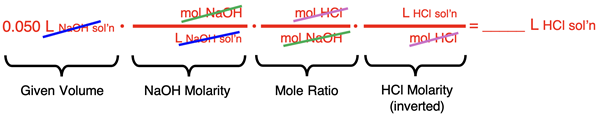
(If necessary, review factor label approach on our Solution Stoichiometry page.)
Once the conversion factors have been set up to cancel units, substitute in numbers. Then use your calculator to determine the answer.

Example 6 - Determining the Molarity of Base
A flask contains 200.0 mL of an NaOH solution of unknown concentration. A student lab group adds 32.5 mL of 0.100 M HCl in order to neutralize it. Determine the molarity of the NaOH solution.
Solution Approach #1
We will show our preferred approach to the problem and then discuss a second approach relying mostly on the factor label method. Both approaches rely on an understanding that the moles of HCl must equal the moles of NaOH at the equivalence point.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
The moles of HCl added to the flask can be determined from the volume and molarity of the acid solution.
# moles HCl = (0.0325 L)•(0.10 mol HCl / 1 L) = 0.00325 mol HCl
This indicates that the flask originally contained 0.00325 moles of NaOH. Knowing the volume of the solution and the moles of NaOH in the solution, the molarity can be calculated.
Molarity NaOH = moles NaOH / Volume of solution
[NaOH] = 0.00325 mol / 0.2000 L = 0.0163 M
(rounded to three significant digits from 0.01625 M)
Solution Approach #2
The second approach relies on the factor label method. We begin with the setup of conversion factors to cancel units and convert from the volume of the HCl solution to the moles of NaOH.
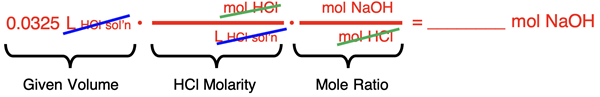
Once the conversion factors are set up, numbers are substituted into the numerators and denominators, and the calculator is used to determine the moles of NaOH in the flask.
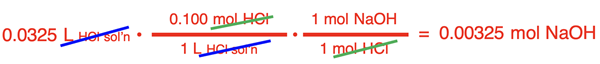
Now that the moles of NaOH in the 200.0 mL of solution are known, the molarity can be calculated.
Molarity NaOH = moles NaOH / Volume of solution
[NaOH] = 0.00325 mol / 0.2000 L = 0.0163 M
(rounded to three significant digits from 0.01625 M)
Next Up
Thus far our discussion of acid-base neutralization has involved the strictest definition: the reaction of an acid with a base of equal strength. We have focused on the reactions between a strong acid and a strong base. On the next page of Lesson 4 - Neutralization Reactions Involving a Weak Acid or Base, we will discuss situations in which the acid and base are of unequal strength. We will present a broader definition of acid-base neutralization and explain how the two models differ. Before plowing forward, it’s always a good idea to do some practice and reinforcement.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. According to the strictest definition of an acid-base neutralization reaction, which of the following are considered neutralization reactions? Select all that apply.
- Reaction of a strong acid with a weak base.
- Reaction of a weak acid with a strong base.
- Reaction of a strong acid with a strong base.
2. In general, what are the products of the reaction of a strong acid with a strong base?
3. A step-by-step method was given for writing the molecular equation for the neutralization reaction of a strong acid with a strong base. Use the method to write the balanced equation for the reaction of ...
a. HClO
4 with NaOH
b. HI with Ba(OH)
2
c. HBr with Ca(OH)
2
4. Write the molecular equation, the complete ionic equation, and the net ionic equation for the neutralization reaction of HI with KOH. (Use H
3O
+ instead of H
+.)
5. Complete the following statements by entering a numerical value in the blank.
- To neutralize 0.050 mol HBr, ________ mol of KOH must be added to it.
- To neutralize 0.050 mol H2SO4, ________ mol of Ba(OH)2 must be added to it.
- To neutralize 0.050 mol HCl, ________ mol of Ca(OH)2 must be added to it.
- To neutralize 0.050 mol HClO4, ________ mol of NaOH must be added to it.
- To neutralize 0.050 mol H2SO4, ________ mol of LiOH must be added to it.
6. A KOH solution is made by dissolving 2.8 g KOH in 0.500 L of water. (Molar mass of KOH is 56 g/mol.)
- Determine the moles of HCl that must be added to the solution for complete neutralization.
- Determine the moles of H2SO4 that must be added to the solution for complete neutralization.
7. A flask contains 45.8 mL of 0.244 M NaOH. It will be neutralized by the addition of 0.236 M HCl.
- How many moles of NaOH are in the flask?
- How many moles of HCl must be added to the flask to neutralize the NaOH?
- What volume of the HCl solution must be added to achieve neutralization?
8. After a recent battery acid spill in South's Autos garage, Mr. Lebrum (Chemistry Teacher) was contacted for help. Mr.Lebrum sent in the "HasMath" team from his AP Chemistry class. Upon arrival at the scene, they found an estimated 18.6 mL of 4.78 M H
2SO
4 waiting for them on the garage floor. In an awesome display of Chemistry for Better Living, the "HasMath" team went to work calculating the moles of sodium bicarbonate (NaHCO
3) powder required to neutralize the spill. (NOTE: 2 moles of NaHCO
3 will neutralize 1 mole of H
2SO
4.) Determine …
- … the moles of H2SO4 present on the garage floor.
- … the moles of NaHCO3 required to neutralize the spilled acid.
- ... (and to really impress the growing crowd of bystanders) the mass of NaHCO3 that must be poured on the spill to neutralize it.
Next Part of this Lesson: Neutralization Reactions Involving a Weak Acid or Base
Jump to Next Lesson: Predicting and Explaining the Acidity Level of Salts
View: Chapter Contents