Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: LeChatelier's Principle
Part a: Disturbances and Stress
Part a: Disturbances and Stress
Part b:
Predicting the Direction of Shift
What is a Disturbance?
We learned in Lesson 2 that reversible reaction systems always achieve equilibrium. Depending on the initial mix of reactants and products, the forward reaction will occur at a greater or lesser rate than the reverse reaction. This causes concentrations to change. As concentrations change, the rates of the forward and reverse reaction become more and more similar. Eventually, the rate of the forward and reverse reaction are equal and concentrations no longer change.
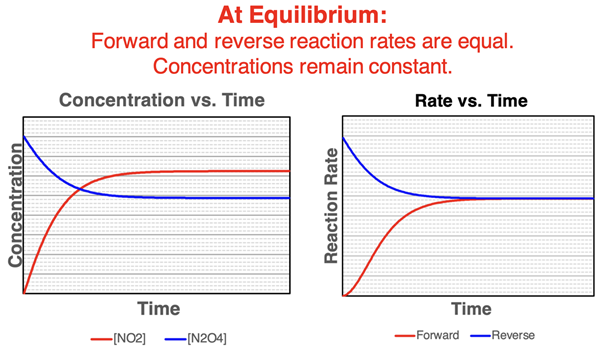
 Once equilibrium is established, the concentrations of reactants and products are fixed. While the two reactions are still occurring, they have no effect upon these concentrations since they occur at the same rate. The Law of Chemical Equilibrium states that the ratio of product to reactant concentrations, each raised to a power equal to its coefficient, is constant. This state will be naturally maintained until an external influence disturbs it. Any externally imposed change in a concentration, a pressure, or a temperature will disturb the equilibrium state. Such disturbances put stress on the system, causing it to readjust until a new equilibrium state with a different set of concentrations is established.
Once equilibrium is established, the concentrations of reactants and products are fixed. While the two reactions are still occurring, they have no effect upon these concentrations since they occur at the same rate. The Law of Chemical Equilibrium states that the ratio of product to reactant concentrations, each raised to a power equal to its coefficient, is constant. This state will be naturally maintained until an external influence disturbs it. Any externally imposed change in a concentration, a pressure, or a temperature will disturb the equilibrium state. Such disturbances put stress on the system, causing it to readjust until a new equilibrium state with a different set of concentrations is established.
In Lesson 3a, we will discuss the variety of disturbances. In Lesson 3b, we will learn how LeChatelier’s Principle explains the response that the system makes to re-establish the equilibrium.
Changes in Concentration
At equilibrium, the K value equals the ratio of product to reactant concentrations (raised to a power equal to their reaction coefficients). Any changes made to the system that changes these concentrations will serve as a disturbance. One common change involves the addition or removal of one of the gaseous or aqueous state reactants or products. Such a change will result in a concentration change and disturb the equilibrium. The reversible system will respond to this stress in a manner that re-establishes the equilibrium.
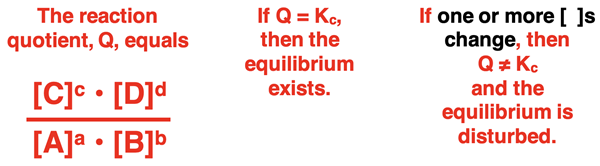
Secondary reactions occurring within the system can often result in the addition or removal of a reactant or product. For instance, the addition of an ion to the system may precipitate another ion that is present in the chemical reaction. This side reaction removes that ion and disturbs the equilibrium. Side reactions can also produce a substance that is present in the reversible reaction system and thus increase its concentration.
Changes in Temperature
At equilibrium, the K value equals the ratio of product to reactant concentrations (raised to a power equal to their reaction coefficients). The value of the equilibrium constant is temperature dependent. If the temperature of the system is changed, the value of K is changed. The result is that the K value and the ratio of product to reactant concentrations are no longer equal. The equilibrium has been disturbed as a result of the temperature-induced change in K value.
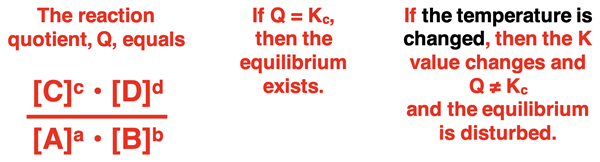
This places a stress on the system to adjust in a manner that re-establishes the equilibrium condition. The manner in which the system responds depends on whether the temperature was increased or decreased. An endothermic process will respond differently than an exothermic process. We will discuss the response in detail in Lesson 3b.
Changes in Pressure (or Volume)
Gaseous reactants and products are sensitive to pressure. Changes in pressure often create a stress on a reversible system that disturbs its equilibrium state. As we learned in our Gases and Gas Laws chapter of this Chemistry Tutorial, the concentration of a gas is dependent upon the pressure of a gas. Increases in pressure cause increases in concentrations of all gaseous reactants and products. This disturbs the equilibrium.
For a pressure to result in a disturbance, there must be an unequal number of moles of gas on opposite sides of the balanced chemical equation. When the number of moles of gas on the reactant side equals the number of moles of gas on the product side, a pressure change will not disturb the equilibrium.

Consider the two reversible systems above. The system on the left consists of four moles of gas on the reactant side and two moles of gas on the product side. A change in pressure would disturb its equilibrium and place a stress on the system to re-establish it. The details of the stress response will be discussed in Lesson 3b. The system on the right consists of two moles of gas on each side of the reaction arrow. A change in pressure would have no effect upon its equilibrium.
Next Up
LeChatelier’s principle describes how reversible reaction systems respond to a disturbance. On the next page of Lesson 3, we will discuss this principle with numerous examples and practice opportunities. But before you click ahead, take some time with the Check Your Understanding questions to internalize your understanding of this page.
Before You Leave
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on LeChatelier’s Principle. Save it to a safe location and use it as a review tool. The Study Card addresses topics from both pages of Lesson 3. (Coming Soon.)
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Each of the following statements describe a change. Answer Yes or No to Does the change cause a disturbance of the equilibrium?
a. The temperature of the system is decreased.
b. A catalyst is added to the system.
c. For the 2 NO
2(g) ⇄ N
2O
4(g) system, the volume of the container is decreased.
d. An inert, non-reactive gas is added to a system of all gases.
e. One of the two gaseous products is removed from the system.
f. Additional solid reactant is added to the system.
g. One of the aqueous state products is precipitated by the addition of a cation.
2.
TRUE or
FALSE:
The addition of aqueous state reactants will disturb the equilibrium by increasing the rate of the forward reaction.
3. Which of the following reversible systems will be disturbed from its equilibrium if the pressure is increased? Select all that apply.
a. 2 NO
2(g) ⇄ 2 NO
(g) + O
2(g)
b. Cl
2(g) + 2 Br
-(aq) ⇄ Br
2(l) + 2 Cl
-(aq)
c. H
2(g) + I
2(g) ⇄ 2 HI
(g)
d. H
2S
(g) ⇄ 2 H
2(g) + S
2(g)