Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: A Model of Solutions
Part b: Solubility and Structure
Part a:
What is a Solution?
Part b: Solubility and Structure
Part c:
The Dissolving Process
Part d:
Solubility, Temperature, and Pressure
Part e:
Dissociation of Ionic Compounds
The Big Idea
The ease with which a substance dissolves depends not just on what it is but on how it’s built. In chemistry, a substance’s molecular or ionic structure determines whether it dissolves well in a given solvent.
What is Solubility?
Solubility refers to the maximum amount of solute that can be dissolved in a particular solvent. The solubility in water varies significantly from substance to substance. An ionic compound like sodium nitrate (NaNO3) is very soluble (meaning dissolve-able) in water with more than 90 g of NaNO3 dissolving in 100 mL of water at room temperature. Other substances like calcium carbonate (CaCO3) can barely dissolve in water and are considered insoluble (meaning does not dissolve). At room temperature and pressure, a maximum of 0.008 moles of methane gas (CH4) will dissolve in 100 mL of water. Under the same conditions, 0.38 moles of methane, nearly 50 times as much, will dissolve in 100 mL of hexane (C6H14). In Lesson 1b, we will learn what makes a solute soluble in a particular solvent.
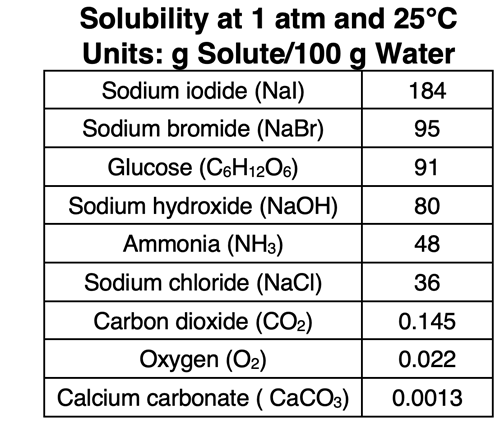
 Like Dissolves Like
Like Dissolves Like
One of the catch phrases regarding solubility is that like dissolves like. A solute will dissolve in a solvent if they have experience similar types of intermolecular forces (IMFs). Using the rule, we would predict that a polar solute will dissolve in a polar solvent like water. A nonpolar solute will dissolve in a nonpolar solvent like hexane (C6H14). We would also predict that nonpolar solutes would not dissolve in polar solvents like water. Similarly, polar solutes seldom dissolve in nonpolar solvents. The concept of polarity becomes critical to predicting solubility. The polarity or nonpolarity of a molecule (and the presence of O-H, N-H, and F-H bonds) determines what type of IMFs the molecule experiences. And because polarity pertains to how molecules are structured, the topic of solubility pertains to molecular structure.
Propane, commonly used in outdoor gas grills, is a hydrocarbon. Hydrocarbons contain nonpolar C-C and C-H bonds. The  intermolecular forces that hold hydrocarbon molecules together are London dispersion forces. Hexane (C6H14), also a hydrocarbon, is a great solvent for other compounds that are nonpolar. Yet hexane has a limited ability to dissolve polar substances. In contrast to hexane, water is a polar molecule. Water molecules are held together in the liquid state by hydrogen bonding, a particularly strong type of dipole-dipole interaction. Water is an effective solvent for polar solutes that are also held together by dipole-dipole interactions or experience hydrogen bonding forces.
intermolecular forces that hold hydrocarbon molecules together are London dispersion forces. Hexane (C6H14), also a hydrocarbon, is a great solvent for other compounds that are nonpolar. Yet hexane has a limited ability to dissolve polar substances. In contrast to hexane, water is a polar molecule. Water molecules are held together in the liquid state by hydrogen bonding, a particularly strong type of dipole-dipole interaction. Water is an effective solvent for polar solutes that are also held together by dipole-dipole interactions or experience hydrogen bonding forces.
Intermolecular Forces and Solubility
The table below displays a variety of compounds, their molecular polarity, the intermolecular force (IMF) that is most dominant between its molecules, and their solubility in water and hexane. It is quite evident from Rows 1-5 of that water is a great solvent for polar solutes. And it is quite evident from Rows 6-11 that hexane is a great solvent for nonpolar solutes. These rows give meaning to like dissolves like.
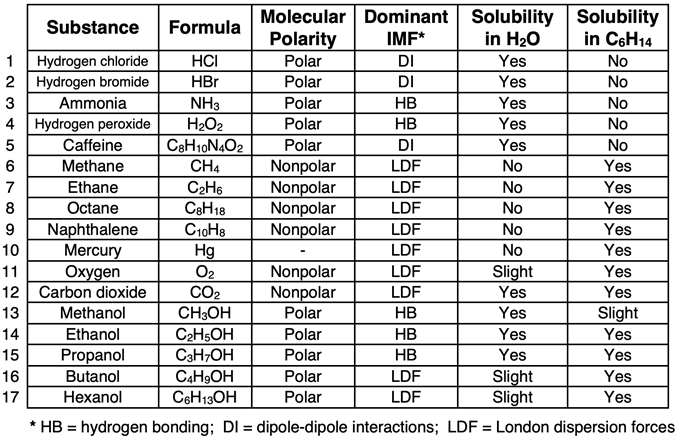
Like dissolves like is a guideline for predicting the solubility of a particular substance in a solvent like water or hexane. However, it should never be regarded as a rule. The behavior of chemical substances is often more easily explained than it is predicted. This is certainly the case for solubility. Pondering the data from Rows 12 to 17 will help us to build a better model of solubility that goes further than like dissolves like.
As seen in Row 12, carbon dioxide (CO2) is a nonpolar molecule with high solubility in hexane. Based on our simple rule, we might expect it to be insoluble in water. Yet CO2 is soluble in water. Those of us who love carbonated beverages can be thankful that it is. There are a couple of reasons for the solubility of CO2 in water. First, as CO2 dissolves in  water, it reacts chemically with water to form carbonic acid. This removes the CO2 from the aqueous solution, allowing additional CO2 to take its place. Second, even though CO2 is nonpolar due to its linear structure, there is a slight interaction of its electronegative O atoms with water molecules. An oxygen atom of CO2 experiences a dipole-dipole-like interaction with the hydrogen atom of water. This interaction is prevalent enough to contribute to the unexpectedly higher solubility of nonpolar CO2 in polar water.
water, it reacts chemically with water to form carbonic acid. This removes the CO2 from the aqueous solution, allowing additional CO2 to take its place. Second, even though CO2 is nonpolar due to its linear structure, there is a slight interaction of its electronegative O atoms with water molecules. An oxygen atom of CO2 experiences a dipole-dipole-like interaction with the hydrogen atom of water. This interaction is prevalent enough to contribute to the unexpectedly higher solubility of nonpolar CO2 in polar water.
Rows 13 to 17 contrasts the interaction of five different alcohols (hydrocarbon chains with an O-H group replacing an H atom). The O-H group of each alcohol is the polar part of the molecule. The hydrocarbon chain, consisting of nonpolar C-C and C-H bonds, is the nonpolar part of the molecule. The hydrocarbon chains of two side-by-side molecules will interact through London dispersion forces. The O-H groups on two side-by-side molecules will interact through hydrogen bonding.
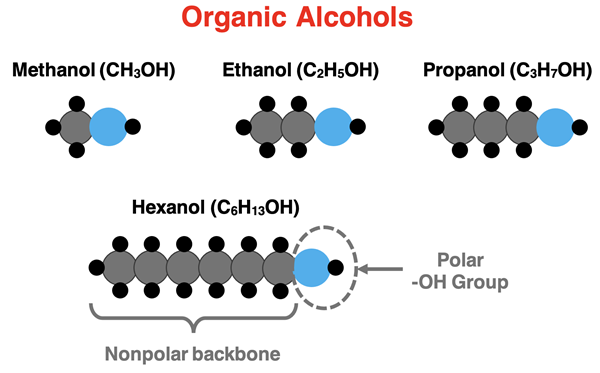
As the size of the hydrocarbon chain gets longer, the London dispersion forces become the more dominant intermolecular force. Methanol (Row 13), with a single carbon atom, interacts with water molecules through hydrogen bonding and is very soluble in water. On the other hand, hexanol (Row 17), with a six-carbon atom chain, interacts with hexane through London dispersion forces and is very soluble in hexane. For these alcohols, like dissolves like becomes a guiding principle when looking more closely at the parts of the molecule.
 How Does Soap Work?
How Does Soap Work?
You’ve been told countless times to wash your hands with soap. With soap is the critical part of the command. It is not enough to wash your hands with water because water can only be expected to attract, dissolve, and wash away polar solutes. And most of what we wish to cleanse ourselves of - grease, oils, germs, etc. - is nonpolar. So how do you entice water to wash away nonpolar molecules? You use soap.
Soap is a complex molecule that consists of a highly polar, hydrophilic end attached to a long, nonpolar, hydrophobic chain. Hydrophilic means water loving. The hydrophilic end consists of polar C-O bonds, explaining their love for water. This part of the molecule is referred to as the head of the molecule. The rest of the molecule consists of a long chain of carbon atoms with attached hydrogen atoms. This is the nonpolar tail of the molecule. Consisting of nonpolar C-C and C-H bonds, the tail will have nothing to do with water. But this also means that the tail is a down-and-dirty lover of grease and oils.
The most common soap is sodium stearate - NaC17H35COO. When the soap dissolves in water, the stearate ion (C17H35COO-) enters the aqueous solution.
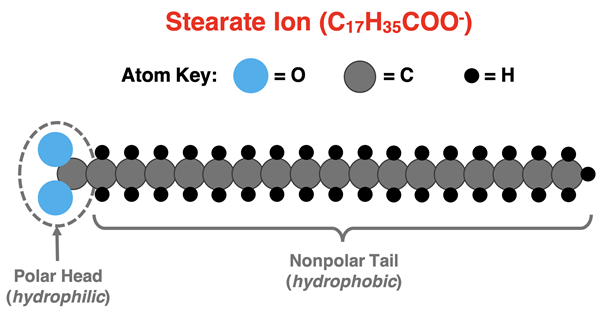
 The hydrophilic end of the stearate ion, containing the polar C-O bonds, is attracted to water. The hydrophobic end is attracted to the nonpolar substance (the dirt) on your hands. In solution, the stearate ions from a micelle that surrounds the grease and oil. A micelle can be thought of as a three-dimensional, spherical structure that forms a cage around the dirt. The nonpolar tails are pointed towards the dirt while the polar head faces outward towards the water. The tail attracts, adheres to, and traps the dirt while the head is attracted to the water. The micelle and the trapped dirt take a ride with the water straight the drain. And that’s Chemistry for Better Living!
The hydrophilic end of the stearate ion, containing the polar C-O bonds, is attracted to water. The hydrophobic end is attracted to the nonpolar substance (the dirt) on your hands. In solution, the stearate ions from a micelle that surrounds the grease and oil. A micelle can be thought of as a three-dimensional, spherical structure that forms a cage around the dirt. The nonpolar tails are pointed towards the dirt while the polar head faces outward towards the water. The tail attracts, adheres to, and traps the dirt while the head is attracted to the water. The micelle and the trapped dirt take a ride with the water straight the drain. And that’s Chemistry for Better Living!
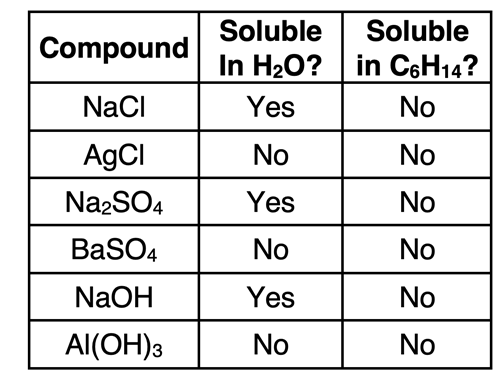
Solubility of Ionic Compounds
Ionic compounds do not consist of molecules. Rather they consist of cations and anions held together by strong ionic bonds in a crystal lattice. Many ionic compounds readily dissolve in water. Others are considered insoluble in water. But none of them, whether they dissolve in water or not, dissolve in nonpolar solvents.
The solubility of ionic compounds in water is explained by the ion-dipole interaction between the ions of the crystal lattice and polar water molecule. The positive pole of the water molecules attracts, surrounds, and stabilizes the anion of the compound. The negative pole of the water molecules stabilizes the cation of the compound. Nonpolar solvents like hexane lack a dipole and are unable to stabilize the cation and anion in solution. We will discuss the aqueous solutions of ionic compounds in greater detail in the next part of Lesson 1 and in even more detail in Lesson 1e.
 You will be tempted to quote “like dissolves like” as the answer to any question of the form why does solute X dissolve in solvent Y? This indicates that you were listening (or reading); but it doesn’t necessarily indicate that you understand solubility. Understanding solubility requires that you understand and give attention to molecular structure and intermolecular forces. It also requires that you understand the mechanism that occurs when a solute is dissolved in a solvent. If structure and IMFs explain the why of solubility, then the mechanism is the how of solubility. In the next part of Lesson 1, we will discuss how things dissolve. But before you leave this page to learn more, take some time to solidify the ideas by following one or more of the suggestions in the Before You Leave section.
You will be tempted to quote “like dissolves like” as the answer to any question of the form why does solute X dissolve in solvent Y? This indicates that you were listening (or reading); but it doesn’t necessarily indicate that you understand solubility. Understanding solubility requires that you understand and give attention to molecular structure and intermolecular forces. It also requires that you understand the mechanism that occurs when a solute is dissolved in a solvent. If structure and IMFs explain the why of solubility, then the mechanism is the how of solubility. In the next part of Lesson 1, we will discuss how things dissolve. But before you leave this page to learn more, take some time to solidify the ideas by following one or more of the suggestions in the Before You Leave section.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Download our Study Card on Solubility and Structure. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding of Solubility and Structure
Use the following questions to assess your understanding of solubility, structure, and intermolecular forces. Tap the Check Answer buttons when ready.
1. Throughout Lesson 1b, the prototype polar solvent is ___________ and the prototype nonpolar solvent is ___________.
a. hexane, water
b. water, hexane
2. Like dissolves like means that _____________.
a. Two identical substances will dissolve in each other.
b. Two substances with the exact same composition of atoms will dissolve in each other.
c. Two substances that are held together by similar types of forces will dissolve in each other.
3.
TRUE or
FALSE:
Like dissolves like is a hard and fast rule for predicting what solutes would dissolve in certain solvents. There are never exceptions or surpises to this rule.
4. Think about this one:
Substance A is polar. Substance B is nonpolar. Substance C dissolves in Substance A. Substance D does not dissolve in Substance A. All four substances are molecular compounds.
Give your best predictions for the following situations. Explain each answer.
- Will Substance B dissolve in Substance A?
- Will Substance C dissolve in Substance B?
- Will Substance D dissolve in Substance B?
- Will Substance D dissolve in Substance C?
5. Did you know that some vitamins that our bodies need dissolve in water and others do not? Those that dissolve in water are excreted through our urinary tract each day if our body doesn’t use them. Those that don’t dissolve in water will be stored in the fat tissue of our bodies if our body doesn’t need them. The water-soluble vitamins are the B vitamins and vitamin C. The fat-soluble (water-
insoluble) vitamins are vitamins A, D, E, and K. Use this information to answer the following questions:
- The B vitamins and vitamin C are ___________. (polar, nonpolar)
- Vitamins A, D, E, and K are ___________. (polar, nonpolar)
- The vitamins that you must be sure to take on a daily basis are vitamins ________ (B and C, A-D-E-and-K)
- Taking too much of vitamins _______________ (B and C, A-D-E-and-K) could be dangerous because they can reach toxic levels in our bodies.
- Taking mega-doses of vitamins _______________ (B and C, A-D-E-and-K) would be senseless and wasteful if our body isn’t actually using them.
6. Elemental mercury is a liquid. As indicated in the table, it dissolves in hexane but not in water. Propose a reason why this is.
7. The following compounds either dissolve in water and not hexane or in hexane and not water. For each case, indicate which solvent it would dissolve in and explain why.
- Carbon disulfide, CS2
- Hydrogen fluoride, HF
- Sodium fluoride, NaF
- Carbon tetrachloride, CCl4
- Boron trichloride, BCl3