Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Acid and Base Dissociation
Part b: Dissociation Constants
Part a:
Strong vs. Weak Acids and Bases
Part b: Dissociation Constants
The Big Idea
Dissociation constants (Ka and Kb) are equilibrium constants that measure the strength of acids and bases. This lesson explores how Ka and Kb values relate to acid-base strength, hydronium ion concentration, and pH, using diagrams, tables, and calculations.
What is a Dissociation Constant?
 Strong and weak acids were introduced in Lesson 3a (Strong and Weak Acids and Bases). Strong acids are acids that completely dissociate into ions when dissolved in water. For every 1 mole of a strong acid dissolved in water, 1 mole of H3O+ ions will be produced. Weak acids only partially dissociate. If 1 mole of a weak acid is dissolved in water, only a small portion of that weak acid will dissociate and produce H3O+ ions. The exact portion that does dissociate is determined by the dissociation constant for that particular acid.
Strong and weak acids were introduced in Lesson 3a (Strong and Weak Acids and Bases). Strong acids are acids that completely dissociate into ions when dissolved in water. For every 1 mole of a strong acid dissolved in water, 1 mole of H3O+ ions will be produced. Weak acids only partially dissociate. If 1 mole of a weak acid is dissolved in water, only a small portion of that weak acid will dissociate and produce H3O+ ions. The exact portion that does dissociate is determined by the dissociation constant for that particular acid.
We represent the dissociation of a weak acid with a generic formula of HA by a reversible reaction arrow:
HA(aq) + H2O(l) ⇄ H3O+(aq) + A-(aq)
The weak acid is part of a reversible system with a forward and a reverse reaction. For weak acids, the reverse reaction is the more dominant reaction and the equilibrium position lies on the reactant side. That is, the reactant concentrations are relatively large and the product concentrations are relatively small.
Every reversible system is described by an equilibrium constant K. For weak acid dissociation reactions, we refer to this K value as the acid dissociation constant and represent it by the symbol Ka. Applying the Law of Chemical Equilibrium discussed in Chapter 14 of our Chemistry Tutorial, we can relate the Ka value to equilibrium concentrations of the acid and its ions:
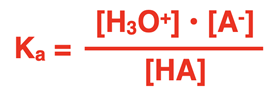
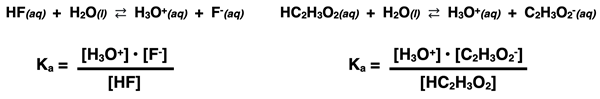
Like any K expression, the acid dissociation constant equation is always written with the concentrations of products in the numerator and the concentration of reactants in the denominator. The Ka expressions for the dissociation of hydrofluoric acid (HF) and acetic acid (HC2H3O2) are shown below.
Ka and Acid Strength
For any reversible system, the equilibrium constant indicates the extent to which the reaction occurs in the forward direction. The K value is the ratio of product concentrations to reactant concentrations at equilibrium. So for a reaction that proceeds strongly in the forward direction, the product concentrations are large, the reactant concentrations are small, and the K value is much greater than 1. For a reaction that does not proceed strongly in the forward direction, the product concentrations are small, the reactant concentrations are large, and the K value is much less than 1. This is the case for weak acids.

For weak acid dissociation, the value of Ka indicates the extent to which dissociation occurs. Every weak acid has its own unique Ka value. The Ka values are less than 1, consistent with partial dissociation. The larger that the Ka value is, the more that the weak acid dissociates. The Ka value of nitrous acid (HNO2) is 5.6x10-4. The Ka value of hydrocyanic acid (HCN) is 6.2x10-10. The considerably larger Ka value of HNO2 means that it dissociates to a greater extent than HCN. While HNO2 is a weak acid, it is a stronger weak acid than HNO2. At equilibrium, the concentration of the ions will be greater in an HNO2 solution compared to an HCN solution
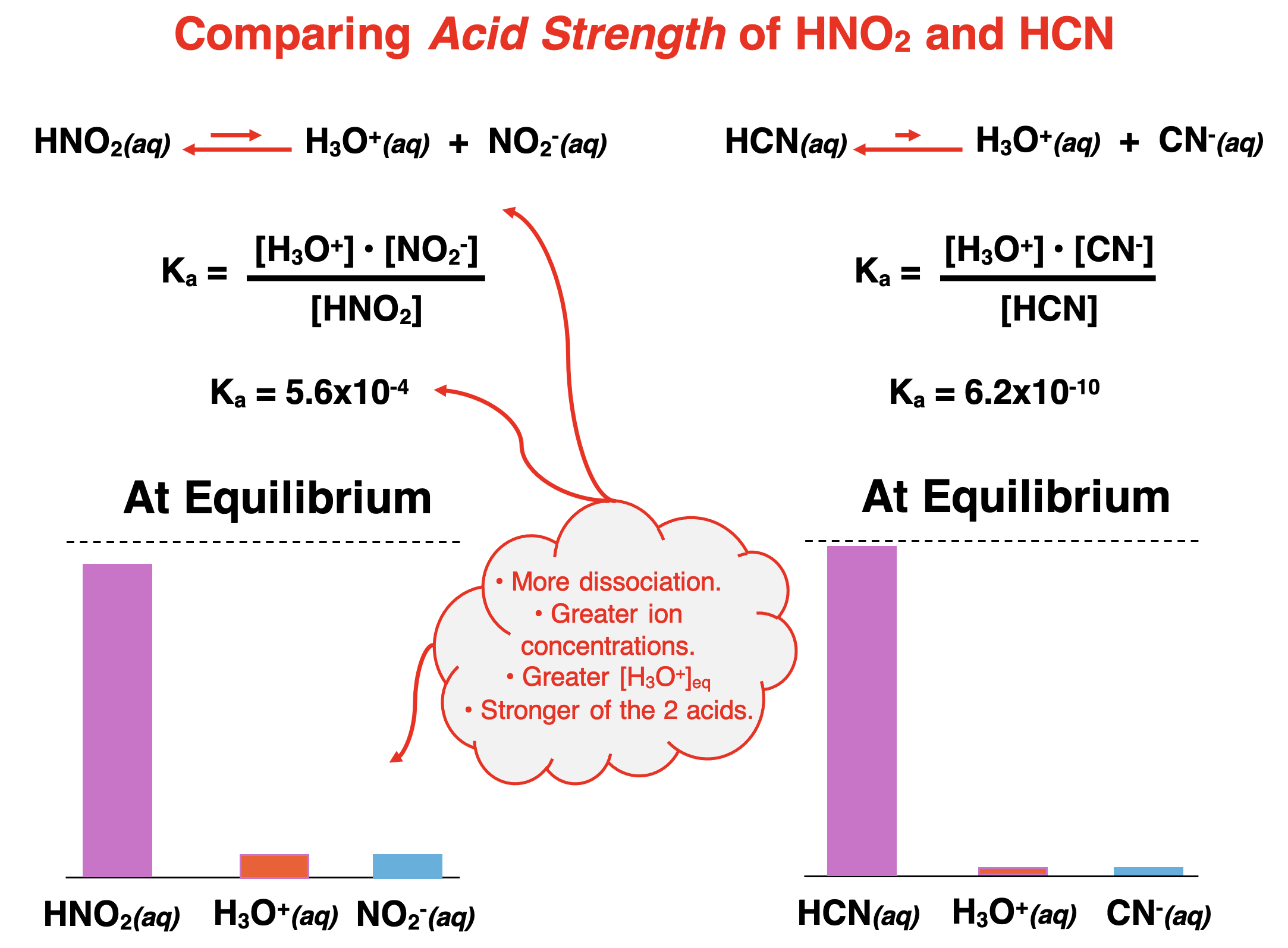
The general rule is that the larger the Ka value is for a weak acid, the more that the acid dissociates into ions, and the stronger that the acid is.

Typical Weak Acids and Their Ka Values
The table below displays Ka values for the more commonly encountered weak acids. The table is organized by Ka value. Acids with the higher Ka values are located at the top of the table. Thus, the weak acids are ordered by acid strength.
| Name of Acid |
Formula |
Ka Value (@25°C) |
|
| Chlorous acid |
HClO2 |
1.1 × 10–2 |
Strongest |
| Nitrous acid |
HNO2 |
7.2 × 10–4 |
|
| Hydrofluoric acid |
HF |
6.6 × 10–4 |
| Formic acid |
HCO2H |
1.78 × 10−4 |
| Acetic acid |
HC2H3O2 |
1.8 × 10–5 |
| Propionic acid |
HC3H5O2 |
1.3 × 10–5 |
| Hypochlorous acid |
HClO |
2.9 x 10-8 |
| Hydrocyanic acid |
HCN |
6.2 x 10-10 |
Weakest |
Additional examples of weak acids and their K
a values can be found in the
Reference section of our
Chemistry Tutorial -
Dissociation Constants.
What are Polyprotic Acids?
The acids in the table above are called monoprotic acids. They each contain one ionizable H
+ ion. Some acids are
polyprotic acids. They may contain two or three ionizable H
+ ions. Carbonic acid, H
2CO
3, is an example of a
diprotic acid that partially dissociates. It has two ionizable H
+ ions. The ions are removed in a stepwise fashion as shown.
H2CO3(aq) + H2O(l) ⇄ H3O+(aq) + HCO3-(aq) Ka1 = 4.5x10-7
HCO3-(aq) + H2O(l) ⇄ H3O+(aq) + CO32-(aq) Ka2 = 4.7x10-11
A diprotic acid typically has two K
a values, one for each dissociation step. They are distinguished from each other as K
a1 and K
a2.
Phosphoric acid, H
3PO
4, is a common
triprotic acid. It has three ionizable H
+ ions that are removed in three dissociation steps as shown.
H3PO4(aq) + H2O(l) ⇄ H3O+(aq) + H2PO4-(aq) Ka1 = 7.1x10-3
H2PO4-(aq) + H2O(l) ⇄ H3O+(aq) + HPO42-(aq) Ka2 = 6.3x10-8
HPO42-(aq) + H2O(l) ⇄ H3O+(aq) + PO43-(aq) Ka3 = 2.4x10-12
The most common polyprotic acid is probably sulfuric acid, H
2SO
4. Sulfuric acid is unique among polyprotic acids because it is considered a strong acid. The first H
+ ion ionizes completely, and the conjugate base, the HSO
4- ion, is formed. The second H
+ ion is removed by the partial dissociation of the HSO
4- ion. The entire process is represented as
H2SO4(aq) + H2O(l) → H3O+(aq) + HSO4-(aq) Strong Acid
HSO4-(aq) + H2O(l) ⇄ H3O+(aq) + SO42-(aq) Ka2 = 1.1x10-2
The table below lists several polyprotic acids and the K
a values for their dissociation steps.
| Name of Acid |
Formula |
Ka1 |
Ka2 |
Ka3 |
| Ascorbic acid |
H2C6H6O6 |
7.9 x 10-5 |
1.6 x 10-12 |
-- |
| Boric acid |
H3BO4 |
5.8 x 10-10 |
1.8 x 10-13 |
1.6 x 10-14 |
| Carbonic acid |
H2CO3 |
4.4 x 10-7 |
4.7 x 10-11 |
-- |
| Citric acid |
H3C6H5O7 |
7.4x 10-4 |
1.7 x 10-5 |
4.1 x 10-7 |
| Oxalic acid |
H2C2O4 |
5.4 x 10-2 |
6.5 x 10-5 |
-- |
| Phosphoric acid |
H3PO4 |
7.1 x 10-3 |
6.3 x 10-8 |
4.2 x 10-13 |
| Sulfuric acid |
H2SO4 |
-- |
1.2 x 10-2 |
-- |
| Sulfurous acid |
H2SO3 |
1.3 x 10-2 |
6.3 x 10-8 |
-- |
Additional examples of polyprotic acids and their K
a values can be found in the
Reference section of our
Chemistry Tutorial -
Dissociation Constants.
Calculations of Ka of a Weak Acid from pH
The K
a of a weak acid can be determined by measuring the pH value of a known concentration of the acid. The pH that results from the weak acid’s dissociation allows one to determine the hydronium ion concentration - [H
3O
+]. This concentration is equal to the anion concentration. The K
a expression can be used to calculate the K
a for the weak acid. The following step-by-step procedure will be helpful.
Step-by-Step Procedure
- Write the balanced chemical equation for the dissociation of the weak acid into its ions.
- Write the Ka expression based on the equation written in Step 1.
- Use the pH to calculate [H3O+] at equilibrium. Visit our pH Mathematics page (if needed).
- The [H3O+]equilibrium = [anion]equilibrium.
- Assume that the [acid]initially = [acid]equilibrium. That is, assume that so little acid dissociates that is concentration is not affected by the dissociation.
- Substitute all equilibrium concentrations into the Ka expression from Step 2 and calculate the Ka value.
NOTE: We will discuss weak acid dissociation problems in greater detail in
Chapter 16 of our
Chemistry Tutorial. We will provide a more detailed background to the above procedure in that chapter and also discuss numerous variations of weak acid and base dissociation problems.
Example 1 - Calculating Ka from pH
A 2.5 M aqueous solution of a weak acid is measured to have a pH of 3.8. Determine the K
a of the acid.
Solution
Step 1: HA(aq) + H2O(l) ⇄ H3O+(aq) + A-(aq)
Step 2: Ka = [H3O+]•[ A-] / [HA]
Step 3: [H3O+] = 10-pH = 10-3.8 = 1.58489... x10-4 M (no rounding until the final calculation)
Step 4: [A-] = 1.58489... x10-4 M
Step 5: [HA] = 2.5 M
Step 6: Concentrations from Steps 3,4, and 5 are substituted into the Ka expression to solve for Ka.
Ka = (1.58489... x10-4 M)•( 1.58489... x10-4 M) / (2.5 M) = 1.0 x 10-8 M
(rounded from 1.00475 x 10-8 M)
Example 2 - Calculating Ka from pH
A 2.5 M aqueous solution of a weak acid is measured to have a pH of 5.8. Determine the K
a of the acid.
Solution
Step 1: HA(aq) + H2O(l) ⇄ H3O+(aq) + A-(aq)
Step 2: Ka = [H3O+]•[ A-] / [HA]
Step 3: [H3O+] = 10-pH = 10-5.8 = 1.58489... x10-6 M (no rounding until the final calculation)
Step 4: [A-] = 1.58489... x10-6 M
Step 5: [HA] = 2.5 M
Step 6: Concentrations from Steps 3,4, and 5 are substituted into the Ka expression to solve for Ka.
Ka = (1.58489... x10-6 M)•( 1.58489... x10-6 M) / (2.5 M) = 1.0 x 10-12 M
(rounded from 1.00475 x 10-12 M)
Effect of Ka on the pH of Solution
Analysis of the two calculation results from Examples 1 and 2 reveals an important pattern. The two examples involved two different acids (different K
a values) having the same initial concentration. Their pH was different because of the differences in their K
a values. Here’s a summary of the results.
As can be seen from the data, the acid with the larger K
a value (Example 1) dissociated to form a solution with a higher hydronium ion concentration. The resulting pH was thus a lower pH value. The stronger the acid is, the more it dissociates to form H
3O
+, and the lower that the pH of the solution will be.
Do Strong Acids Have Ka Values?
Yes, they do. And the K
a values of strong acids are always much greater than 1.0. This indicates that the reaction favors the production of products. The molar concentration of undissociated HA molecules is significantly less than the concentration of dissociated ions. For our purposes, we simply consider strong acid dissociation reactions as proceeding to completion . This is why we represent the dissociation of a strong acid by a forward reaction arrow (as opposed to the reversible reaction arrow, ⇄).
HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
For strong acids, we would approximate the concentration of H
3O
+ in solution to be equal to the concentration of the acid before it dissociated.
Dissociation constants for a variety of strong acids are shown in the table below.
| Name of Acid |
Formula |
Ka Value |
| Perchloric acid |
HClO4 |
Very Large |
| Hydroiodic acid |
HI |
3.2 × 109 |
| Hydrobromic acid |
HBr |
3.2 × 109 |
| Hydrochloric acid |
HCl |
1.3 × 106 |
| Sulfuric acid |
H2SO4 |
1.0 × 103 |
| Chloric acid |
HClO3 |
5.0 x 102 |
| Nitric acid |
HNO3 |
2.4 × 101 |
Base Dissociation and Kb Values
Like a weak acid, a weak base partially dissociates in aqueous solutions to produce its conjugate acid and hydroxide ions (OH
-). A base with the generic formula B will undergo the following dissociation reaction:
B(aq) + H2O(l) ⇄ BH+(aq) + OH-(aq)
The extent to which this reaction occurs is reflected by the K
b value of the base.
Kb is referred to as the
base dissociation constant. Every base has its own unique K
b value. A large K
b value (much greater than 1) indicates that the dissociation is complete and the conjugate acid and hydroxide ions are the predominant
species in the solution. For weak bases, the K
b values are less than 1.0, indicating partial dissociation.
Ammonia, NH
3, has a K
b value of 1.8x10
-5. As was the case for acids, the dissociation constant indicates the ratio of product concentrations to reactant concentrations. The chemical equation for the dissociation of NH
3 in water and the K
b expression are:
As a second example, methyl amine, CH
3NH
2, is a weak base with a K
b value of 4.4x10
-4. The chemical equation for the dissociation of CH
3NH
2 in water and the K
b expression are:
(Need to review? Check out our pages on
Base Dissociation Equations and
Writing K Expressions as needed.)
Common Weak Bases and Their Kb Values
The table below displays K
b values for the more commonly encountered weak bases. The table is organized by K
b value with bases having the higher K
b values being located at the top of the table. Thus, the weak base are ordered by base strength.
| Name of Base |
Formula |
Kb Value (@25°C) |
|
| Piperidine |
C5H10NH |
1.3× 10–3 |
Strongest |
| Trimethyl amine |
(CH3)3N |
1.0 × 10–3 |
|
| Ethyl amine |
C2H5NH2 |
5.6 × 10–4 |
| Methyl amine |
CH3NH2 |
4.4 × 10–4 |
| Ammonia |
NH3 |
1.8 × 10–5 |
| Hydrazine |
NH2NH2 |
1.7 × 10–6 |
| Pyridine |
C5H5N |
1.8 × 10–9 |
| Aniline |
C6H5NH2 |
4.3 × 10–10 |
Weakest |
Additional examples of weak bases and their K
b values can be found in the
Reference section of our
Chemistry Tutorial -
Dissociation Constants.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Dissociation Constants. Save it to a safe location and use it as a review tool.
- Check out our Reference section page with concepts related to Ka and Kb values for strong and weak acids, weak bases, and polyprotic acids. When you need some data, you’ll likely find it in our Reference section.
Check Your Understanding of Dissociation Constants
Use the following questions to assess your understanding of Ka and Kb values. Tap the Check Answer buttons when ready.
1. Complete the following paragraph:
A strong acid _______________ (partially, completely) dissociates while a weak acid _______________ (partially, completely) dissociates. The dissociation of the _________ (strong, weak) acid is best modeled as a reversible system with a dominant _________________ (forward, reverse) reaction. The dissociation of a ____________ (strong, weak) acid is considered to proceed to completion. A solution of a weak acid will consist primarily of ___________________ (undissociated acid molecules, dissociated ions) whereas a solution of a strong acid will consist primarily of ___________________ (undissociated acid molecules, dissociated ions).
2. The acid dissociation constant for a weak acid is ____________.
- relatively large, much greater than 1
- relatively small, much less than 1
- .. come on now! This is Chemistry, not fortune telling. Do you expect things to be that predictable.
For
Questions #3-6: Formic acid (HCO
2H) has a K
a value of 1.8 x 10
-4 and hypochlorous acid (HClO) has a K
a value of 2.9x10
-8.
3. Which acid has the greatest concentration of undissolved acid at equilibrium?
- formic acid (HCO2H)
- hypochlorous acid (HClO)
4. Which acid has the greatest concentration of hydronium ions at equilibrium?
- formic acid (HCO2H)
- hypochlorous acid (HClO)
5. Which acid has the greatest pH value at equilibrium?
- formic acid (HCO2H)
- hypochlorous acid (HClO)
6. Which is the stronger of the two acids?
- formic acid (HCO2H)
- hypochlorous acid (HClO)
7. Acid A and B are both weak acids. Acid A has the larger K
a value. Which of the following statements are true of acid A? (Assume their concentrations are equal.) Select all that apply.
- Acid A is the stronger acid.
- Acid A has a higher pH value.
- Acid A dissociates into ions more than acid B.
- Acid A is more concentrated with H3O+ than acid B.
For
Questions #8-10: Pyridine (C
5H
5N) has a K
b value of 1.8 x 10
-9 and ammonia (NH
3) has a K
b value of 1.8 x 10
-5.
8. Which base is the stronger base?
- pyridine (C5H5N)
- ammonia (NH3)
9. Which base has the greater tendency to dissociate into ions?
- pyridine (C5H5N)
- ammonia (NH3)
10. Which base has the greater pH value at equilibrium?
- pyridine (C5H5N)
- ammonia (NH3)