Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Equilibrium
Part c: Calculations of K and Concentration
Part a:
The Equilibrium State
Part b:
Equilibrium Constant Expressions
Part c: Calculations of K and Concentration
Part d:
Predicting the Direction of Reaction
Part e:
Analyzing Equilibrium Systems
The Big Idea
In this lesson, you’ll learn how to compute the equilibrium constant (K) from measured concentrations and then rearrange equilibrium expressions to solve for unknown concentrations in a reversible reaction.
The Kc Equation
We discussed the Law of Chemical Equilibrium on the previous page of Lesson 2. The law states that at any given temperature, a reversible reaction system is at equilibrium if the ratio of product to reactant concentrations, each raised to a power equal to its coefficient, is constant. Consider the generic equation for a reaction between A and B to produce C and D. The coefficients are a, b, c, and d.
aA + bB ⇄ cC + dD
The system will proceed towards equilibrium until the ratio
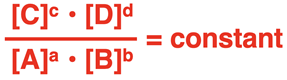
is a constant value. Once reached, the concentrations will no longer change. If the concentrations are measured at equilibrium, then the equation can be used to determine the value of the equilibrium constant (Kc). This value will be the same value for this reaction regardless of the starting conditions and provided that the temperature is not changed.
On this page of Lesson 2, we will learn how to use the law to calculate an equilibrium constant. We will also learn how to calculate an unknown equilibrium concentration if the Kc value and the other equilibrium concentrations are known.
Calculating Kc
The process of calculating the Kc value involves the following steps:
- Carefully write out the equilibrium constant equation. (Use our Tutorial page as a reference if you need to.)
- Identify the equilibrium concentrations of each gas or aqueous state substance in units of mol/L or M. (These values are often given.)
- Substitute concentration values into the appropriate location of the equation.
- Use your calculator to solve for the Kc value. Be sure to use the appropriate exponents. And be sure to divide by all denominator concentrations.
Let’s look at three examples of varying difficulty to see how the K
c value can be calculated from the equilibrium concentrations.
Example 1 – Kc Calculation
Consider the following reversible reaction system.
CO(g) + H2O(g) ⇄ CO2(g) + H2(g)
The following set of equilibrium concentrations have been measured:
[CO] = 0.102 M
[H2O] = 0.144 M
[CO2] = 0.328 M
[H2] = 0.257 M
Calculate the value of K
c for this reaction.
Solution:
The K
c equation is first written (below). Concentrations are given in mol/L. These values are carefully substituted into the K
c equation. A calculator is used to calculate the value of K
c. The answer is rounded to the third significant digit. The units in the denominator cancel the units in the numerator; this K
c value has no units.
Example 2 – Kc Calculation
Consider the following reversible reaction system.
2 HI(g) ⇄ H2(g) + I2(s)
The following set of equilibrium concentrations have been measured:
[HI] = 2.589 M
[H2] = 0.012 M
Calculate the value of K
c for this reaction.
Solution:
The K
c equation is first written; the solid product I
2 is not included in the K
c expression since it is a solid. Concentrations are given in mol/L. These values are carefully substituted into the K
c equation. A calculator is used to calculate the value of K
c. The answer is rounded to the second significant digit. There is an uncancelled unit of mol/L in the denominator of the fraction. This is simplified to L/mol.
Example 3 – Kc Calculation
Consider the following reversible reaction system.
2 NO(g) + 2 H2(g) ⇄ N2(g) + 2 H2O(g)
The following set of equilibrium concentrations have been measured:
[NO] = 0.119 M
[H2] = 0.105 M
[N2] = 0.425 M
[H2O] = 0.388 M
Calculate the value of K
c for this reaction.
Solution:
The K
c equation is first written. Concentrations are given in mol/L. These values are carefully substituted into the K
c equation. A calculator is used to calculate the value of K
c. Be sure to include the exponents. The answer is rounded to the third significant digit. There is an uncancelled unit of mol/L in the denominator of the fraction. This is simplified to L/mol.
Calculating Kp
As discussed in Lesson 2b, homogeneous gas phase systems often express the equilibrium constant in terms of partial pressures of the reactants and products. This constant is denoted by the symbol K
p and is a different value than the K
c value for the same reaction. For the generic reaction between A and B to produce C and D:
aA(g) + bB(g) ⇄ cC(g) + dD(g)
The
pressure equilibrium constant equation is
Measurements of the partial pressures of gaseous reactant and products at equilibrium allow one to determine the K
p value. The process of doing so is similar to what has been previously done with the K
c calculations.
Example 4 – Kp Calculation
Consider the following reversible reaction system.
S2Cl2(g) + Cl2(g) ⇄ 2 SCl2(g)
At a certain temperature, the equilibrium pressures of reactants and products are …
Partial pressure of S2Cl2 = 1.27 atm
Partial pressure of Cl2 = 1.33 atm
Partial pressure of SCl2 = 3.39 atm
Calculate the equilibrium constant value (K
p).
Solution:
The K
p equation is first written. Partial pressures are given in atm. These values are carefully substituted into the K
p equation. A calculator is used to calculate the value of K
p. The answer is rounded to the third significant digit. The units in the denominator cancel the units in the numerator; this K
p value has no units.
Calculating an Equilibrium Concentration ( [ ]eq )
The equilibrium constant equation can be used to calculate an unknown equilibrium concentration of a reactant or product if the other equilibrium concentrations are known and the K
c value is known. The process for doing so involves the following steps:
- Carefully write out the equilibrium constant equation. (Use our Tutorial page as a reference if you need to.)
- Identify all known information – the Kc value and any known equilibrium concentrations of any gas or aqueous state substance in units of mol/L or M.
- Perform the proper algebraic steps to isolate the unknown concentration by itself on one side of the equation and all other variables on the other side of the equation. This new equation is set up to solve for the unknown.
- Substitute known values into the appropriate location of the new equation.
- Use your calculator to solve for the unknown value. Be sure to use the appropriate exponents. And be sure to divide by all denominator values.
Examples 5 and 6 demonstrate the use of this process. Additional practice ideas are provided in
the Before You Leave section near the end of this page.
Example 5 – [ ]eq Calculation
Consider the following reversible reaction:
FeO(s) + CO(g) ⇄ Fe(s) + CO2(g)
The equilibrium constant (K
c) for the reaction is 0.778. The equilibrium concentration of CO is 0.561 M. What is the equilibrium concentration of CO
2?
Solution:
The K
c equation is first written; the solid reactant and product are not included in the K
c expression.
The K
c value and the concentration of the denominator are given. The equation needs to be rearranged to solve for [CO
2]. Multiplying both sides of the equation by [CO] will produce a [CO
2] = … equation. Known values are carefully substituted into the equation. A calculator is used to calculate the value of [CO
2]. The answer is rounded to the third significant digit.
Example 6 – [ ]eq Calculation
Consider the following reversible system:
2 N2O(g) + 3 O2(g) ⇄ 4 NO2(g)
At a certain temperature, the equilibrium constant (K
c) is 2.06 L/mol. Equilibrium concentrations of the two reactants are …
[N2O] = 0.330 M
[O2] = 0.500 M.
Calculate the equilibrium concentration of NO
2.
Solution:
This problem represents slightly more difficult algebra. The problem begins the same way - the K
c equation is first written.
The K value and the concentration of the reactants (in denominator) are given. The equation needs to be rearranged to solve for [NO
2]. Multiplying both sides of the equation by the denominator of the right side will isolate the [NO
2]
4 variable. Then taking the 4
th root of each side will produce the [NO
2] = … equation. The last algebraic step can be replaced by raising both sides to the ¼-th power; this has the equivalent effect of removing the power from the [NO
2].
Known values are carefully substituted into the equation. A calculator is used to calculate the value of [NO
2]. The answer is rounded to the third significant digit. Depending on the calculator you are using, this may not be as routine of a step as stated. If you are having difficulty, it would be wise to first calculate everything inside the parenthesis; be careful with the exponents. Then raise the result to the 0.25 power (or take the fourth root).
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding of K Calculations
Use the following questions to practice your skill at calculating K values or concentration values. Tap the Check Answer buttons when ready.
1. Consider the reaction:
Br2(g) + 5 F2(g) ⇄ 2 BrF5(g)
The following equilibrium concentrations are measured:
[Br2] = 0.0516 M
[F2] = 0.601 M
[BrF5] = 0.0225 M
Calculate the equilibrium constant value (K
c).
2. The following reversible system
2 N2O(g) + 3 O2(g) ⇄ 4 NO2(g)
is observed to have equilibrium concentrations of …
[N2O] = 0.112 M,
[O2] = 0.311 M, and
[NO2] = 0.470 M.
Calculate the equilibrium constant value (K
c).
3. Consider the following reversible reaction system.
2 NaHCO3(s) ⇄ Na2CO3(s) + CO2(g) + H2O(g)
Once equilibrium is established, the two gaseous products have pressures of ...
Partial pressure of CO2 = 1.45 atm
Partial pressure of H2O = 1.11 atm
Calculate the value of the pressure equilibrium constant (K
p).
4. Consider the reversible reaction:
Br2(g) + 5 F2(g) ⇄ 2 BrF5(g)
At a certain temperature, the equilibrium constant (Kc) is 0.432 L4/mol4.
The following equilibrium concentrations are measured:
[Br2] = 0.0390 M
[F2] = 0.584 M
Calculate the equilibrium concentration of BrF
5.