Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Acid-Base Equilibria
Part d: Determination of the pH of Salts
Part a:
Weak Acid and Weak Base Dissociation
Part b:
Percent Dissociation and Ka and Kb
Part c:
Dissociation of Polyprotic Acids
Part d: Determination of the pH of Salts
Part e:
Common Ion Effects and Buffers
Part f:
Titration Analysis
The Big Idea
Determining the pH of salt solutions involves understanding hydrolysis, the relationship between Ka and Kb, and the use of an ICE table to relate equilibrium concentrations to an equilibrium constant value.
What is Hydrolysis?
A salt is an ionic compound consisting of a cation (a positive ion) and an anion (a negative ion). When a salt dissolves in water, it dissociates into its ions. Often times, the ions of a salt can react with water, either by donating a proton to water or accepting a proton from water. Since these reactions produce hydronium (H3O+) or hydroxide ions (OH-), they affect the pH of the solution. In acid-base chemistry, these reactions of a salt with water are referred to as hydrolysis.
The hydrolysis of salts was discussed in great detail in Chapter 15 of our Chemistry Tutorial. On this page, we will take that discussion further by discussing how to determine the pH of a solution containing a salt that undergoes hydrolysis. We assume an understanding of hydrolysis in our discussion. Those who need to establish a sturdy background understanding of the topic should visit our page titled Predicting and Explaining the Acidity Level of Salts.
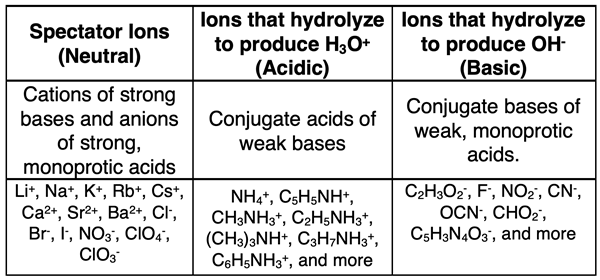
How to Predict the Ions that Hydrolyze
Some ions of salts do not hydrolyze. These include ions that are the cations of strong bases or the anions of strong, monatomic acids. Such ions behave as spectator ions in aqueous solutions.
Some ions of salts hydrolyze by donating a proton to water to produce H3O+. These include ions that are the conjugate acids of weak bases. An example would include the ammonium ion, NH4+. It is the conjugate acid of NH3, a weak base. The ammonium ion is present in salts such as NH4Cl and NH4NO3. When either of these salts dissociate, the ammonium ion separates from the anion and then undergoes hydrolysis:
NH4+(aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
Some ions of salts hydrolyze by accepting a proton from water to produce OH-. These include ions that are the conjugate bases of weak acids. An example would include the fluoride ion, F-. It is the conjugate base of HF, a weak acid. The fluoride ion is present in salts such as NaF and KF. When either of these salts dissociate, the fluoride ion separates from the cation and then undergoes hydrolysis:
F-(aq) + H2O(l) ⇄ HF(aq) + OH-(aq)
A summary of these ideas is shown in the table below. Further discussion and elaboration can be found on our Chapter 15 page titled Predicting and Explaining the Acidity Level of Salts.
Determining the K Values for Hydrolysis Reactions
Hydrolysis reactions are reversible reactions with equilibrium positions that typically lie on the reactant side. Like any acid-base equilibrium system, the determination of the pH requires a knowledge of the equilibrium constant. An ion that hydrolyzes by donating a proton to water is acting as an acid. Its hydrolysis reaction is described by a Ka value. An ion that hydrolyzes by accepting a proton from water is acting as a base. Its hydrolysis reaction is described by a Kb value.
The Ka and Kb values for hydrolysis reactions can be determined by identifying the conjugate of the ion that hydrolyzes. An ion such as NH4+ that acts as an acid is the conjugate of the weak base NH3. The Ka value for the hydrolysis of NH4+ is determined by the equation
Ka of NH4+ = 1.0 x 10-14 / Kb of NH3
An ion such as F- that acts as a base is the conjugate of the weak acid HF. The Kb value for the hydrolysis of F- is determined by the equation
Kb of F- = 1.0 x 10-14 / Ka of HF
The table below includes other examples of ions that hydrolyze and the logic for determining the K values for its hydrolysis reaction.
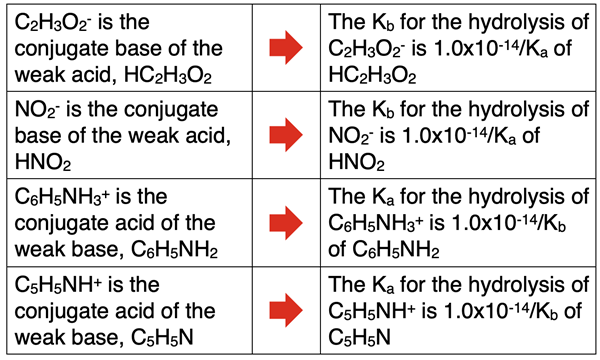
As an example calculation, let’s determine the Kb value of the acetate ion, C2H3O2-. The Ka of its conjugate acid (HC2H3O2) is 1.8x10-5. Therefore,
Kb of C2H3O2- = 1.0 x 10-14 / 1.8 x 10-5 = 5.6 x 10-10
As a second example calculation, let’s determine the Ka value of the anilinium ion, C6H5NH3+. The Kb of its conjugate base (C6H5NH2) is 7.1x10-10. Therefore,
Ka of C6H5NH3+ = 1.0 x 10-14 / 7.1x10-10 = 1.4 x 10-9
In all these relationships, the 1.0x10-14 value is the ion product constant (Kw) of water. The Kw value is temperature dependent. We assume a temperature of 25°C throughout this chapter.
Those needing a more detailed discussion of how to determine the K value of a hydrolysis reaction can learn more on our Chapter 15 page titled Ka and Kb Values of Conjugate Acid-Base Pairs.
How to Determine the pH of a Salt Solution
The process of determining the pH of a salt solution involves the following steps:
- If given the formula (or the name) of the salt, identify the two ions that it contains. (Need help? Visit Identifying the Ions in an Ionic Compound.)
- Determine which of the two ions will hydrolyze. If neither hydrolyzes, then the pH is 7.0. (Need help? Visit What Ions are Acidic or Basic in Aqueous Solutions?)
- Write the balanced chemical equation for the hydrolysis of the ion. One of the products will be either H3O+ or OH-. (Need help? Visit Hydrolysis of Salts.)
- Calculate the Ka or Kb for the hydrolysis reaction. (Need help? See Determining K Values of Hydrolysis Reactions.)
- Write the equilibrium constant equation that relates the K value to the equilibrium concentrations of the ions. (Need help? Visit Writing K Expressions.)
- Set up an ICE table to determine expressions for the equilibrium concentrations. These expressions will usually include an unknown - x. Use the Five-Percent Rule if necessary.
- Substitute the equilibrium expressions into the equilibrium constant equation from Step 5 and use algebra to solve for x. Check to ensure that the Five-Percent Rule is a valid assumption.
- Use the ICE table to relate the value of x to either [H3O+] or [OH-]. Use this concentration value to determine the pH. (Need help? Visit Calculting the pH from Hydronium Ion Concentration.)
We will demonstrate the use of this strategy in Examples 1 and 2. There are additional practice problems with complete solutions in the
Check Your Understanding section.
Example 1 - Determining the pH of a 0.50 M NH4Cl(aq)
Determine the pH of an aqueous solution of 0.50 M NH
4Cl. (NH
3 is a weak base with a K
b value of 1.8x10
-5.)
Solution
Step 1: NH
4Cl is a salt that contains ammonium ions (NH
4+) and chloride ions (Cl
-).
Step 2: The NH
4+ is the conjugate acid of a weak base (NH
3). It will hydrolyze by donating a proton to water. The Cl
- is a spectator ion; it is the anion of a strong acid (HCl).
Step 3: The balanced equation for the hydrolysis reaction is:
NH4+(aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
Step 4: The K
a for this reaction is
Ka of NH4+ = 1.0 x 10-14 / 1.8 x 10-5 = 5.6 x 10-10
(rounded from 5.555555 ... x10-10)
Step 5: The equilibrium constant equation for this hydrolysis reaction is:
5.6 x 10-10 = [NH3] • [H3O+] / [NH4+]
Step 6: The completed ICE table is shown below. The [NH
4+]
initial is equal to the concentration of the salt. The product concentrations are initially 0. A +x and a -x can be used for the change in concentration. The equilibrium concentration is always the initial concentration plus the change in concentration. We assume that 0.50 - x ≈ 0.50. After determining x, we will check the validity of the assumption using the Five-Percent Rule.
Step 7: Concentration expressions from the Equilibrium row of the ICE table can be substituted into the equilibrium constant equation (step 5). (The unrounded value of K
a is used in the solution; we will round our final answer to two significant digits.) The algebraic steps for determining x are shown:
5.555555 ... x10-10 = [NH3] • [H3O+] / [NH4+]
5.555555 ... x10-10 = x• x / 0.50
2.777777... x 10-10 = x2
x = √ (2.777777... x 10-10)
x = 1.666666... x 10-5
(The value of x is less than 5% of the 0.50 value. Thus, the assumption that 0.50 - x ≈ 0.50 is a valid assumption.)
Step 8: The value of x is defined in the Equilibrium row of the ICE table as the [H
3O
+]. This concentration value can be used to determine the pH. The work is shown here.
x = [H
3O
+] = 1.666666... x 10
-5 M
pH = -log(1.666666... x 10
-5)
pH = 4.78
Example 2 - Determining the pH of a 0.50 M NaF(aq)
Determine the pH of an aqueous solution of 0.50 M NaF. (HF is a weak acid with a K
a value of 6.6×10
-4.)
Solution
Step 1: NaF is a salt that contains sodium ions (Na
+) and fluoride ions (F
-).
Step 2: The F
- is the conjugate base of a weak acid (HF). It will hydrolyze by accepting a proton from water. The Na
+ is a spectator ion; it is the cation of a strong base (NaOH).
Step 3: The balanced equation for the hydrolysis reaction is:
F-(aq) + H2O(l) ⇄ HF(aq) + OH-(aq)
Step 4: The K
b for this reaction is
Kb of F- = 1.0 x 10-14 / 6.6×10-4 = 1.5 x 10-11
(rounded from 1.5151515 ... x10-11)
Step 5: The equilibrium constant equation for this hydrolysis reaction is:
1.5 x 10-11 = [HF] • [OH-] / [F-]
Step 6: The completed ICE table is shown below. The [F
-]
initial is equal to the concentration of the salt. The product concentrations are initially 0. A +x and a -x can be used for the change in concentration. The equilibrium concentration is always the initial concentration plus the change in concentration. We assume that 0.50 - x ≈ 0.50. After determining x, we will check the validity of the assumption using the Five-Percent Rule.
Step 7: Concentration expressions from the Equilibrium row of the ICE table can be substituted into the equilibrium constant equation (step 5). (The unrounded value of K
b is used in the solution; we will round our final answer to two significant digits.) The algebraic steps for determining x are shown:
1.5151515 ... x10-11 = [HF] • [OH-] / [F-]
1.5151515 ... x10-11 = x• x / 0.50
7.575757 ... x 10-12 = x2
x = √ (7.575757 ... x 10-12)
x = 2.752409... x 10-6
(The value of x is less than 5% of the 0.50 value. Thus, the assumption that 0.50 - x ≈ 0.50 is a valid assumption.)
Step 8: The value of x is defined in the Equilibrium row of the ICE table as the [OH
-]. This concentration value can be used to determine the pH. The work is shown here.
x = [OH-] = 2.752409... x 10-6 M
[H3O+] = 1.0 x 10-14 / (2.752409... x 10-6)
[H3O+] = 3.633180... x 10-9 M
pH = -log(3.633180... x 10-9 M)
pH = 8.44
A Subtle Point Regarding Water’s Self-Ionization
We learned in Chapter 15 that
water undergoes self-ionization to produce hydronium ions (H
3O
+) and hydroxide ions (OH
-).
H2O(l) + H2O(l) ⇄ H3O+(aq) + OH-(aq)
At 25°C, the self-ionization of water produces concentrations of H
3O
+ and OH
- of 1.0x10
-7 M.
When a salt is dissolved in water, the initial concentrations of these ions is not technically 0. It is 1.0x10
-7 M. This is a very small concentration but on occasion it matters. The completion of the ICE table can account for this by listing 1.0x10
-7 M as the initial concentration of these ions.
This complicates the mathematics and the usual simplification employs using the Five-Percent Rule. In this case, we assume that x will be much larger than 1.0x10
-7 and that
1.0x10-7 + x ≈ x
That is, we assume that water’s self-ionization makes a negligible contribution to the equilibrium value of [H
3O
+]. This simplification is valid if x turns out to be at least 20 times larger than 1.0x10
-7. This is equivalent to saying that the neglected 1.0x10
-7 is less than 5% of x.
In all our problems, we have assumed the contribution of water’s self-ionization to the equilibrium concentrations of H
3O
+ and OH
- to be negligible. And in our problems, the value of x turns out to be more than 20 times larger than 1.0x10
-7 M, thus satisfying the Five-Percent Rule and validating our assumption. In situations in which the initial salt concentration is considerably smaller, the contribution of water’s self-ionization to the equilibrium concentrations of H
3O
+ and OH
- becomes more significant.
Practice Makes Perfect
As with any problem-solving skill in Chemistry, it is important to put the skill into practice. See the suggestions in the
Before You Leave section for some practice and reinforcement opportunities.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Consider an aqueous solution of 0.420 M KNO
2. (KOH is a strong base. HNO
2 is a weak acid with a K
a value of 4.00 x 10
-4).
- The pH of the solution will be affected by the _____ ion.
Choices: K+ ion or NO2- ion
b. The reaction that occurs after KNO
2 dissociates is …
Choice A: K+(aq) + H2O(l) ⇄ KOH(aq) + H+(aq)
Choice B: NO2-(aq) + H2O(l) ⇄ HNO2(aq) + OH-(aq)
c. The equilibrium constant of the selected reaction is ______.
d. Determine the equilibrium concentration of OH
-. Use an ICE table.
e. Determine the pH of the solution.
2. Use an ICE table to calculate the pH of a 2.4 M KCN solution. The K
a of HCN is 6.20 x 10
-10.
3. Use an ICE table to calculate the pH of a 0.25 M KClO solution. The K
a of HClO is 3.50 x 10
-8.
4. Use an ICE table to calculate the pH of a 2.5 M solution of (CH
3)
2NH
2Cl. The K
b of (CH
3)
2NH is 5.40 x 10
-4.